Dermatology for Animals, Stafford Heights, QLD; School of Veterinary Science, University of Queensland, QLD, Australia
There is a range of diagnostic procedures that can easily be conducted in a consult room which, whilst requiring minimal equipment, give an enormous amount of information in the management of dermatology cases.
Microscope Setup
There are 2 basic cytology samples that can be examined using a microscope. Those that require some form of stain to identify the organism of interest and those that are unstained. Each requires the microscope to be set up in a different way that allows the easy identification of the target organisms.
1. Unstained cytology
This may be used to examine the hair shaft or look for various mites. The microscope must be set to increase the natural contrast of the sample. This can be achieved in a number of ways:
a. Using phase contrast if the microscope has this facility.
b. Phase can be mimicked by closing the iris on the condenser or by lowering the condenser away from the microscope stage.
The sample is placed on a glass slide and a coverslip is placed over the top, which has the effect of creating an even thickness to the sample and therefore an even plane of focus for examination.
2. Stained cytology
This is used to examine all other types of cytology whether the target of interest is some microorganism, an inflammatory cell or fine-needle aspirate. The material collected has no colour and cannot be seen until a range of stains is used to highlight it. In contrast to the previous cytology, the microscope is set up with the iris open, the condenser level with the bottom of the microscope stage or the phase contrast disengaged.
Diagnostic Procedures
Unstained Cytology
1. Coat Combing
The coat is brushed with a flea comb and the resultant scurf examined for surface parasite identification. With some parasites (particularly Cheyletiella), concentration of the scurf via emulsion in faecal float solution may be necessary.
Parasites identified include:
 Cheyletiella
Cheyletiella
 Lice
Lice
 Fleas
Fleas
 Trombicula
Trombicula
2. Skin Scraping
 Superficial
Superficial
 The area to be sampled is lightly clipped, paraffin oil is placed on the skin and a blunt scalpel is used to collect surface scale and crust. The aim is not to cause capillary bleeding as the parasites being collected are in the superficial layers of the skin.
The area to be sampled is lightly clipped, paraffin oil is placed on the skin and a blunt scalpel is used to collect surface scale and crust. The aim is not to cause capillary bleeding as the parasites being collected are in the superficial layers of the skin.
 Once the material is collected onto glass slides, it is covered with a coverslip (to make an even plane of focus), and the condenser is lowered or the iris closed (to increase the contrast) so that a normal microscope behaves like a phase contrast microscope.
Once the material is collected onto glass slides, it is covered with a coverslip (to make an even plane of focus), and the condenser is lowered or the iris closed (to increase the contrast) so that a normal microscope behaves like a phase contrast microscope.
 The following parasites may be identified:
The following parasites may be identified:
 Sarcoptes
Sarcoptes
 Cheyletiella
Cheyletiella
 Demodex cornei
Demodex cornei
 Demodex gatoi
Demodex gatoi
 Trombicula
Trombicula
 Lynxacarus radovsky
Lynxacarus radovsky
 Dermanyssus gallinae
Dermanyssus gallinae
 Deep
Deep
 This involves a deeper level of scrape - until capillary oozing is seen. This is done whenever Demodex is suspected. But as there is no such thing as a 'classic' look to demodicosis, it should be a routine test method in most investigations.
This involves a deeper level of scrape - until capillary oozing is seen. This is done whenever Demodex is suspected. But as there is no such thing as a 'classic' look to demodicosis, it should be a routine test method in most investigations.
 The area to be sampled is clipped (#40 blade) and then the skin is squeezed (increases mite yields), paraffin oil is applied and a blunt scalpel is used to scrape until capillary oozing (i.e., not bleeding from laceration) is evident. The material is transferred to a glass slide and examined as above. NB the feet are often difficult to scrape and will bleed before an adequate depth is reached. Hair plucks may be performed and the base of the hair examined for the mites. If scraping the feet, move to an erythematous area at the edge of the affected area rather than the more swollen, severely affected skin. If the scrapes are negative, a biopsy is sometimes necessary to establish the diagnosis (this is also true for the Shar Pei).
The area to be sampled is clipped (#40 blade) and then the skin is squeezed (increases mite yields), paraffin oil is applied and a blunt scalpel is used to scrape until capillary oozing (i.e., not bleeding from laceration) is evident. The material is transferred to a glass slide and examined as above. NB the feet are often difficult to scrape and will bleed before an adequate depth is reached. Hair plucks may be performed and the base of the hair examined for the mites. If scraping the feet, move to an erythematous area at the edge of the affected area rather than the more swollen, severely affected skin. If the scrapes are negative, a biopsy is sometimes necessary to establish the diagnosis (this is also true for the Shar Pei).
3. Trichogram
A very simple yet powerful technique that allows the following to be assessed:
 Anagen:telogen ratio
Anagen:telogen ratio
 Congenital defects
Congenital defects
 Infections
Infections
 Self-trauma
Self-trauma
 Follicular casts
Follicular casts
 Pigment disturbances
Pigment disturbances
Hair is grasped with either your fingernails or padded mosquito forceps and placed in a drop of oil on a glass slide. A coverslip is placed on top and the hair examined with the microscope set up as above to increase contrast.
Stained Cytology
One of the most powerful investigative techniques is the collection and examination of cytology samples. The following may be identified:
 Bacteria
Bacteria
 Malassezia
Malassezia
 Fungal elements
Fungal elements
 Inflammatory cells
Inflammatory cells
 Acantholytic cells
Acantholytic cells
There are a number of different techniques of collection each useful in its own situation. No one method is better than another, and several may be used in different areas on the same animal.
1. Acetate Tape Preparations
 Particularly where lesions are dry/flaky
Particularly where lesions are dry/flaky
 Interdigital areas
Interdigital areas
 Periocularly
Periocularly
The acetate tape is placed onto the skin surface a number of times in the same place (6 times will remove the entire stratum corneum). It is then placed sticky side down onto a glass slide and stained with the purple stain of a modified Wright's stain (e.g., Diff-Quick). The microscope is set up so that the iris is completely open with the condenser set high. The epithelial debris and any organisms can be clearly seen and emersion oil can be applied to the back of the tape if required (without the need of a coverslip).
NB the tape used must be the old-fashioned clear tape (i.e., not the frosted "magic" or invisible tape).
The material collected with the following techniques is all stained in a similar manner. The material is air dried and may be heat-fixed if necessary. It is then stained with Diff-Quick and examined with the microscope set up as for acetate preparations. Gram stains may also be performed.
2. Direct Smear
 Fluid-containing lesions
Fluid-containing lesions
3. Impression Smear
 Moist/greasy lesions
Moist/greasy lesions
 After crust removed
After crust removed
 Expressing fluid from lesion
Expressing fluid from lesion
 Opening surface of pustule/papule/vesicle
Opening surface of pustule/papule/vesicle
4. Cotton Bud Smear
 Draining tracts
Draining tracts
 Ear canals
Ear canals
 Interdigital
Interdigital
5. Scalpel Blade
 Sample under crust
Sample under crust
 Vesicles
Vesicles
 Peeling St. corneum
Peeling St. corneum
6. Fine-Needle Aspirate
 Nodules, tumours, cysts
Nodules, tumours, cysts
 A 22-ga needle with a 12-ml syringe is used to collect material from the centre of the lesion. The material is aspirated onto a slide, dried and stained as above. Neoplastic cells may be difficult to identify so the help of a clinical pathologist should be sought. Even with high skill levels, tumours should always be diagnosed on the basis of histopathology.
A 22-ga needle with a 12-ml syringe is used to collect material from the centre of the lesion. The material is aspirated onto a slide, dried and stained as above. Neoplastic cells may be difficult to identify so the help of a clinical pathologist should be sought. Even with high skill levels, tumours should always be diagnosed on the basis of histopathology.
Examination for Dermatophytes
1. Woods Lamp Examination
 Warm lamp for 10 min, expose hair for 3–5 min
Warm lamp for 10 min, expose hair for 3–5 min
 Individual hair fluorescence (not the scale or sebum)
Individual hair fluorescence (not the scale or sebum)
 M. canis
M. canis
 Positive in 30–80%
Positive in 30–80%
 False positive: M. distortum, M. audouinii, T. schoenleinii, Corynebacterium, Pseudomonas, medications, keratin, soap, petroleum
False positive: M. distortum, M. audouinii, T. schoenleinii, Corynebacterium, Pseudomonas, medications, keratin, soap, petroleum
 False negative: Iodine destroys reaction
False negative: Iodine destroys reaction
2. Direct Examination of Hair Shafts
The hair shafts that fluoresce may be examined for the presence of arthrospores (fungal elements) at the bulb of the hair. Most veterinary dermatophytes are ectothrix invaders. Examination of the hairs may require chlorphenolac or potassium hydroxide clearing to allow better visualisation of the elements.
3. Fungal Culture
NB only for dermatophytes; if deep mycoses or other fungal infections are suspected, these should be sent to an accredited laboratory for culture because of the occupational health and safety risks.
 Culture medium
Culture medium
 Dermatophyte test medium (DTM)
Dermatophyte test medium (DTM)
 Observe daily
Observe daily
 Dermatophytes
Dermatophytes
 Metabolise protein
Metabolise protein 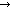 alkaline
alkaline 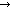 colour change
colour change
 Blastomyces, Sporothrix schenckii, Histoplasma capsulatum, Coccidioides, Pseudoallescheria, some Aspergillus species may cause a change to red in DTM
Blastomyces, Sporothrix schenckii, Histoplasma capsulatum, Coccidioides, Pseudoallescheria, some Aspergillus species may cause a change to red in DTM
 Occasional saprophyte (Scopulariopsis) can
Occasional saprophyte (Scopulariopsis) can 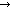 false positive
false positive
 Microscopic examination is essential
Microscopic examination is essential
 Cryptococcus, Candida, Aspergillus and many agents of phaeohyphomycoses are inhibited by cycloheximide 6
Cryptococcus, Candida, Aspergillus and many agents of phaeohyphomycoses are inhibited by cycloheximide 6
 Saprophytes
Saprophytes
 Metabolise CHO
Metabolise CHO 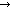 acid
acid  no colour change initially, but once culture is large, there is a sudden (overnight) colour change of the entire test media.
no colour change initially, but once culture is large, there is a sudden (overnight) colour change of the entire test media.
 Sabouraud's dextrose agar
Sabouraud's dextrose agar
 This is used to allow the fungus to form macroconidia more efficiently and allow species identification.
This is used to allow the fungus to form macroconidia more efficiently and allow species identification.
 Used in the following circumstances:
Used in the following circumstances:
 Granulomatous lesions, draining tracts
Granulomatous lesions, draining tracts
 Non/poor response to appropriate antibiotics
Non/poor response to appropriate antibiotics
 Rods present on cytology
Rods present on cytology
Biopsy
A biopsy should be collected for any of the following circumstances:
 Neoplasia/suspected neoplasia
Neoplasia/suspected neoplasia
 Persistent ulceration
Persistent ulceration
 Failure to respond to previous treatment
Failure to respond to previous treatment
 Unusual/serious clinical signs
Unusual/serious clinical signs
 Vesicular dermatosis
Vesicular dermatosis
 Treatment is expensive and/or potentially dangerous
Treatment is expensive and/or potentially dangerous
In general a biopsy should be collected within 3 weeks if a dermatosis is not responding to what appears to be appropriate therapy. Biopsy collection early in the disease process helps avoid nonspecific or masking changes as a result of chronicity. Lesions evolve through a life cycle from early to mature to chronic. The most diagnostic stage is generally early in the process. Chronic changes are commonly nonspecific and nondiagnostic. Early biopsy and definitive diagnosis also allow appropriate therapy to be started.
Site Selection
Selection involves careful examination of the entire animal for the most representative lesions. They should cover the range of spectrum from very early to more mature, but should include the active stages. Including the spectrum of lesions gives far more information and greatly enhances the chance of a diagnosis. For example, if investigating depigmentation, it is essential to biopsy the actively depigmenting lesions (i.e., the grey areas, not the chronic pink areas where a diagnosis is much less likely). Alopecia should be sampled from the centre, junctional and normal skin. Multiple samples should be selected, as this increases the chance of a diagnosis.
Site Preparation
There should be no surgical preparation. Do not scrub or clean the skin in any way as this will remove the surface layers which are often critical for diagnosis.
The only preparation is the gentle removal of hair.
If the lesion is crusted and this crust is lost during sampling, include it in the formalin pot so that it can be cut in. Remember to advise the pathologist that the crust has been included so that it is added to the block for sectioning.
Biopsy Technique
1. Wedge
a. Common for excisional collection of nodules or tumours
b. Vesicular lesions
c. Panniculitis
d. Edge of lesion (allows orientation by the pathologist). Traditionally the lesion is biopsied so that the spectrum, from normal to affected, follows the long axis of the sample. This means that the sample can be sectioned in this plane and represents the graduation of pathology as well.
2. Punch
This is the most commonly used technique. Generally a 6-mm or 8-mm punch is used for most areas, with a smaller (4-mm) punch used for the nose, lips or mouth. The area is clipped of hair and local anaesthetic instilled under the site. The punch is placed firmly on the skin and rotated in one direction whilst continuing to push. This will allow sampling of the entire skin thickness down to the subcutaneous tissue. Fine scissors are used to separate the sample from the subcutis. The tissue is blotted free of blood and placed on a piece of paper and then dropped into the formalin. Placing the sample on a piece of stiff paper or tongue depressor prevents it from curling when placed in the formalin. This is particularly important when collecting thin skin.