Department of Clinical Veterinary Science, University of Bristol, Langford, Bristol, UK
These affect the ability of the blood to coagulate. Coagulation, the process of clot formation, is achieved in vivo by a combination of primary and secondary haemostasis (Stokol 2005). Although clotting disorders usually refer to secondary haemostatic disorders, primary haemostasis will also be discussed briefly due to the close integration and clinical need to differentiate between the two.
Overview of Haemostasis
Primary Haemostasis
This involves platelet adhesion, via Von Willebrand factor (vWF), to the subendothelial collagen, with ensuing activation and aggregation of platelets, resulting in the formation of a platelet plug. When platelets become activated, phosphatidylserine (PS; previously platelet factor 3) becomes exposed on the platelet membrane and acts as a scaffold for the assembly of coagulation factors in secondary haemostasis. Thus primary and secondary haemostasis are closely linked. Primary haemostasis also involves an initial reflex constriction of the blood vessel.
Secondary Haemostasis
This involves formation of fibrin by coagulation factors to stabilise the primary haemostatic plug. Classically secondary haemostasis has been explained by the coagulation cascade which is divided into intrinsic and extrinsic pathways with a final common pathway (Figure 1). This cascade is useful when interpreting diagnostic haemostatic testing but does not reflect how coagulation occurs in vivo. In vivo there is extensive interaction between these pathways with the extrinsic pathway (tissue factor) initiating coagulation and the intrinsic pathway amplifying it. Surface (or contact) activation is required for in vitro clotting tests but is not required for in vivo coagulation.
| Figure 1. | 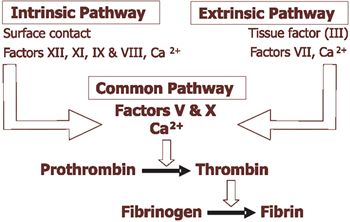
Schematic diagram of secondary haemostasis for test interpretation |
|
| |
Most coagulation factors are protease enzymes which circulate in an inactive form. All coagulation factors are synthesised in the liver. The liver requires vitamin K for the synthesis of Factors II, VII, IX and X.
Tertiary Haemostasis
Tertiary haemostasis consists of fibrinolysis to break down the fibrin clot predominantly via the action of plasmin cleaving fibrin.
Inhibitors
Limiting reactions ensure that clotting is localised to the required area. Substances important include antithrombin (AT) which, in association with heparin, inactivates many of the coagulation factors, and prostacyclin (PGI2), produced by blood vessel endothelial cells, which inhibits platelet aggregation and causes vasodilation.
Approach to Feline Coagulopathies
History and Clinical Findings
Inherited disorders tend to present in younger cats. It is important to find out the response of the cat to any previous trauma or surgery as severe inherited disorders will usually have resulted in bleeding complications. Information regarding any bleeding problems in related animals should be obtained. Recent exposure to toxins such as rodenticides or drugs (e.g., aspirin, NSAIDs) that can affect bleeding should be investigated.
Primary haemostatic disorders are characterised by bleeding from mucosal surfaces (e.g., haematuria, epistaxis), petechiae and prolonged bleeding from cuts or venipuncture. Secondary haemostatic disorders are characterised by more severe bleeding into, e.g., joints and body cavities, haematomas, ecchymoses and delayed bleeding from cuts.
Laboratory Investigation
Blood samples taken for the investigation of bleeding disorders (Table 1) should be collected before starting any therapy. Atraumatic venipuncture, to avoid excessive activation of haemostasis and local consumption of platelets, is required together with appropriate sample handling and submission.
Table 1. Laboratory investigation of bleeding disorders.
|
Haemostatic Stage |
Screening Test |
Component Evaluated |
|
Primary Haemostasis |
Platelet count (in-house estimation from a blood smear*) |
Platelet number |
|
Buccal mucosal bleeding time (BMBT)* |
Platelet (number and function), vessel abnormalities |
|
Secondary Haemostasis |
Activated clotting time (ACT)* |
Intrinsic & common pathways |
|
Activated partial thromboplastin time (APTT) |
Intrinsic & common pathways but more sensitive than ACT |
|
Prothrombin time (PT) |
Extrinsic & common pathways |
|
Thrombin time (TT) |
Common pathway, quantifies fibrinogen levels |
* Tests which can be performed in-house.
Platelet Count Estimation
Automated cell counting machines can struggle to count feline platelets, as described in the talk on Thrombocytopenia. Any thrombocytopenia should be confirmed by examination of a blood smear.
BMBT
Since vessel wall disorders are quite rare, a BMBT in a cat with a normal platelet count is usually a test of platelet function. In cats with coagulation defects the BMBT is usually normal, but rebleeding may occur. A BMBT is carried out under heavy sedation or general anaesthesia using a spring-loaded bleeding time device which makes a pair of standardized incisions in the mucosa. Normal BMBT in cats is <3.3minutes.
ACT
Tubes for measurement of ACT are produced commercially and contain diatomaceous earth to act as the contact activator. Platelet phospholipid is still required for coagulation so ACT is prolonged in severe thrombocytopenia (<10x109/l). Clotting factors must be <10% normal to prolong the ACT. Blood is collected into the tube and the tube inverted and the time taken for complete clot formation recorded. Normal ACT in cats at room temperature is <165 seconds. An ACT is also available on the I-STAT analyser.
APTT and PT
These are usually carried out by commercial laboratories using citrated blood (an accurate ratio of 1:9 sodium citrate:blood is required). Samples (usually cooled plasma) should be delivered to the laboratory as soon as possible and samples from a normal animal may be required as a control. Clotting times >30% prolonged compared to controls are considered abnormal. Individual clotting factors need to be <30% before APTT or PT are prolonged. Point of care coagulation instruments (e.g., SCA2000) can determine the APTT and PT on small amounts of fresh citrated whole blood. These have been shown to be reliable in dogs.
The APTT is more sensitive than the ACT and not dependent on platelets. Since Factor VII (a vitamin K-dependent factor) has the shortest half life of all clotting factors, the PT will prolong before the APTT in cases of vitamin K deficiency or rodenticide toxicity.
TT
Not commonly performed but is prolonged in cases of hypo- or dys-fibrinogenaemia, increased fibrin degradation product levels or circulating heparin as it assesses the formation of fibrin in response to thrombin.
Specific Factor Assays
Deficiency of specific factors can be identified by specialist laboratories although this can be costly. The laboratory should be contacted for submission requirements before the sample is taken.
Encountered Feline Coagulopathy Disorders
Disorders of Primary Haemostasis
Thrombocytopenia, encountered reasonably frequently in cats, is discussed in a later lecture. Inherited thrombocytopathias are rarely described in cats (Chediak-Higashi syndrome in Persians, vWD), but hepatic, renal, neoplastic, infectious (e.g., FIP) diseases, disseminated intravascular coagulopathy (DIC) and drugs (e.g., aspirin) can all cause acquired thrombocytopathias.
Disorders of Secondary Haemostasis
Inherited Disorders: The most common inherited congenital coagulopathy in cats is Factor XII (Hageman) deficiency (Brooks and DeWilde 2006). It is an autosomal recessive disorder (affecting both males and females) that delays in vitro activation of the APTT and ACT, causing these to be prolonged (often markedly), but does not result in bleeding in vivo. It has been reported in the DSH, DLH and other breeds including Siamese and Himalayan. Definitive diagnosis rests on measurement of Factor XII activity. No treatment is required.
Haemophilia A (Factor VIII deficiency) and Haemophilia B (Factor IX deficiency) are sex-linked autosomal recessive (affecting males only) traits. In a recent study (Brooks and DeWilde 2006) Haemophilia A and B were both diagnosed in young cats (<1year) and were found predominantly in DSH cats, although other breeds have been affected e.g., Birman, Himalayan. All haemophilic cats in this report had factor activities of <5% of feline standard plasma and had shown signs of bleeding: subcutaneous or intramuscular haematomas, prolonged bleeding after neutering and gingival bleeding from teeth eruption sites. Haemophilic bleeding tendencies vary depending on the factor activity present. Coagulation testing reveals prolongation of the ACT and APTT, although these (especially ACT) can be normal if the factor deficiency is not severe. Definitive diagnosis depends on measurement of Factor VIII or IX activity.
Other inherited coagulopathies reported in cats include combined Haemophilia A or B and Hageman trait, Factor XI deficiency, and Factor I (fibrinogen) ± XI deficiency in Maine Coons.
Treatment of factor deficiencies involves replacement of the factors required. Fresh whole blood transfusions are useful if anaemia is present. Fresh (within 6 hours of collection) frozen plasma contains active factors. Bleeding cats should not be given intramuscular injections. Avoidance of drugs with can impair haemostasis is also important together with maintaining an atraumatic life. Breeding should be avoided.
A hereditary vitamin K-responsive coagulopathy in Devon Rex cats is associated with moderate to marked decreases in the activity of Factors II, VII, IX and X due to a defective vitamin K metabolism enzyme. It affects both males and females resulting in haematomas, haemarthrosis and body cavity bleeding. APTT and PT are markedly prolonged. These cases respond to oral vitamin K1 at 5mg/kg/d.
Acquired Disorders: Liver disease is an important cause of acquired coagulopathies in cats (Center, et al 2000, Lisciandro, et al 1998) since the liver synthesises clotting factors. Coagulopathies can also arise in cholestatic liver disease in which absorption of the fat soluble vitamin K is impaired due to biliary stasis. Multiple abnormal coagulation times can occur including prolonged PT and APTT. Although abnormal coagulation times are common with liver disease, clinical signs of spontaneous bleeding are rare. Vitamin K1 is helpful (0.5mg/kg SQ BID 2-3 times prior to e.g., surgery, and every 7-21days thereafter) to correct the PT and APTT. Hepatic lipidosis and severe cholangiohepatitis cases can respond well to vitamin K1 (Center, et al 2000).
Vitamin K deficiency also arises due to rodenticide toxicity, although this is less common in cats than dogs (Kohn, et al 2003). Cases present with lethargy, inappetance, haematomas, dyspnoea due to thoracic haemorrhage and/or collapse. The PT prolongs before the APTT. Mild thrombocytopenia may be present. Treatment comprises vitamin K1 (2.5mg/kg SQ 1st day then 0.25-2.5mg/kg PO in divided doses) for at least a week (up to 6weeks), with monitoring of the PT 24 hours after stopping treatment to dictate whether further treatment is required.
Vitamin K-responsive coagulopathies have been reported in cats with malabsorption due to inflammatory bowel disease or exocrine pancreatic insufficiency.
Methimazole, used in the treatment of hyperthyroidism, has been reported to affect activation of vitamin K-dependent factors although one retrospective study found evidence to support this in only one of 20 methimazole-treated cats (Randolph, et al 2000).
DIC arises due to the systemic activation of haemostasis as a result of underlying disease processes in the body. Platelets and factors are involved in widespread clot formation and fibrinolysis is activated. This results in a thrombocytopenia, factor depletion (prolonged PT and APTT), low antithrombin, and increased D-dimers from breakdown of cross-linked fibrin. Cases present with haemorrhage and/or thrombosis. In cats DIC is most commonly associated with neoplasia, liver disease and FIP (Peterson, et al 1995).
FIV infection has been associated with prolongation of the APTT due to an intrinsic pathway problem although the cause of this has not been identified (Hart and Nolte 1994).
References
1. Brooks, M. & DeWilde, L. (2006) Feline Factor XII Deficiency. Compendium on Continuing Education for the Practicing Veterinarian, 28, 148-155.
2. Center, S.A., Warner, K., Corbett, J., Randolph, J.F. & Erb, H.N. (2000) Proteins invoked by vitamin K absence and clotting times in clinically ill cats. Journal of Veterinary Internal Medicine, 14, 292-297.
3. Hart, S.W. & Nolte, I. (1994) Hemostatic disorders in feline immunodeficiency virus-seropositive cats. Journal of Veterinary Internal Medicine, 8, 355-362.
4. Kohn, B., Weingart, C. & Giger, U. (2003) Haemorrhage in seven cats with suspected anticoagulant rodenticide intoxication. Journal of Feline Medicine and Surgery, 5, 295-304.
5. Lisciandro, S.C., Hohenhaus, A. & Brooks, M. (1998) Coagulation abnormalities in 22 cats with naturally occurring liver disease. Journal of Veterinary Internal Medicine, 12, 71-75.
6. Peterson, J.L., Couto, C.G. & Wellman, M.L. (1995) Hemostatic disorders in cats: a retrospective study and review of the literature. Journal of Veterinary Internal Medicine, 9, 298-303.
7. Randolph, J.F., DeMarco, J., Center, S.A., Kantrowitz, L., Crawford, M.A., Scarlett, J.M. & Brooks, M. (2000) Prothrombin, activated partial thromboplastin, and proteins induced by vitamin K absence or antagonists clotting times in 20 hyperthyroid cats before and after methimazole treatment. Journal of Veterinary Internal Medicine, 14, 56-59.
8. Stokol, T. (2005) Disorders of haemostasis. In: BSAVA Manual of Canine and Feline Clinical Pathology (ed. by E. Villiers & L. Blackwood), pp. 83-98. BSAVA, Gloucester.