Theresa W. Fossum, DVM, PhD, DACVS
Tom and Joan Read Chair in Veterinary Surgery, Director, Clinical Programs and Biomedical Devices, Michael E. DeBakey Institute, Professor of Surgery Texas A&M University, College of Veterinary Medicine, College Station, TX, USA
Management of Pleural Effusion
Animals with pleural air or fluid usually exhibit a restrictive respiratory pattern (i.e., rapid, shallow respirations) and they may be extremely dyspneic. Thoracentesis (see below) should be performed prior to taking radiographs in severely dyspneic animals with suspected pleural cavity disease. Removal of even small amounts of pleural effusion or air may significantly improve ventilation, allowing safer manipulation of the patient for radiographic procedures. Most dyspneic animals will allow thoracentesis to be performed with minimal restraint; general anesthetics should be avoided. The animal should be allowed to remain in sternal recumbency and oxygen provided by face mask or nasal insufflation if they will tolerate it. A negative tap does not rule-out pleural effusion; however, if the animal remains dyspneic after thoracentesis, underlying lung disease (i.e., pneumonia, pulmonary edema, pulmonary contusions, pulmonary neoplasia) or loculated fluid should be suspected. Providing nasal oxygen or placing the animal in an oxygen cage may be beneficial while treatment of the pulmonary disease is initiated.
Needle Thoracentesis
Needle thoracentesis is performed with a small gauge (#19 to #23) butterfly needle attached to a 3-way stopcock and syringe, or an over-the-needle catheter attached to an extension tubing, 3 way stopcock, and syringe. The appropriate site for thoracentesis should be selected based on physical examination findings or, if available, radiographic findings. Usually, aspiration of either side of the thorax will adequately drain the contralateral hemithorax since the mediastinum in dogs and cats is thin and permeable to fluid. However, with some diseases, particularly chylothorax and pyothorax, unilateral effusions may occur due to thickening of the mediastinum associated with chronic inflammation.
Perform thoracentesis at the 6th, 7th, or 8th intercostal space, near the level of the costochondral junction. Clip the selected site and perform a local anesthetic block, if needed (rare!). Aseptically prepare the site and introduce the needle into the middle of the selected intercostal space. Use care to avoid the large vessels associated with the posterior aspect of the rib margins. Advance the needle into the pleural space. Aspirate fluid while the needle is being advanced to allow prompt recognition of the appropriate depth of needle placement. With the bevel of the needle facing inward, orient the needle against the rib cage to prevent damage to the lung surface. Gently aspirate fluid and place 5 ml samples in an EDTA tube and clot tube for analysis of cell counts and biochemical parameters, respectively. Additionally, make 6 to 8 direct smears for cytologic evaluation and submit samples for aerobic and anaerobic cultures.
Chest Tube Placement
Chest tube placement should not be attempted in an animal with severe respiratory distress. Generally, stabilization and improved ventilation can first be accomplished by removing some pleural air or fluid via needle thoracentesis. In critically ill patients, chest tubes can occasionally be placed without the use of general anesthesia; local anesthesia (i.e., local anesthetic infiltration or an intercostal nerve block) being sufficient. However, most animals with pleural cavity disease benefit from intermittent positive pressure ventilation and oxygen supplementation during tube placement. When general anesthesia is used, control of the animal's airway (via endotracheal intubation and positive pressure ventilation) and oxygen therapy should be rapidly achieved.
Treatment of pleural cavity disease varies depending on the underlying etiology. For traumatic pneumothorax, intermittent needle thoracentesis may be sufficient in some animals to prevent dyspnea while the lung heals, but chest tubes are occasionally required. However, chest tube placement and continuous drainage of air in animals with spontaneous pneumothorax that have undergone mechanical pleurodesis is recommended to allow pleurodesis. With some types of pleural effusion (i.e., pyothorax), tube thoracentesis and thoracic lavage are mandatory in the primary treatment of most affected animals.
Incorrectly placed or improperly managed chest tubes are extremely dangerous in animals. However, if precautions are taken to assure that the animal cannot remove the tube prematurely, or that if the animal chews on the tube a pneumothorax will not occur, chest tubes simplify the management of some animals with pleural effusion or pneumothorax. The choice of which side to place the chest tube is made by evaluating the radiographs. Occasionally, bilateral chest tubes may be necessary; however, in most dogs and cats the mediastinum is permeable to fluid or air, allowing drainage of both hemithoraxes through a single tube. The exception to this may be in chylothorax or pyothorax.
Components of a tube thoracostomy include a chest tube, an apparatus to connect the tube to a syringe or to a continuous suction bottle, and a device to collect the drained material (syringe or collecting bottle). Commercially available tubes are usually made of polyvinyl chloride or silicone rubber and are less reactive than red rubber feeding tubes. Commercial tubes come with a metal stylet that simplifies tube placement, but may increase the risk of perforating lung tissue when compared to red rubber feeding tubes. The latter are usually inserted using a large hemostat or Carmalt clamp. Commercial chest tubes come in various sizes ranging from 14 to 40 French. The size of the thoracostomy tube should approximate the diameter of the mainstem bronchus; however, smaller tubes may be adequate for removal of air, while larger tubes may required with more viscous effusions. If a commercial tube is used, it can be attached via a five-in-one connector (Christmas tree adaptor) to either a 3-way stopcock or tubing from a continuous suction device. The ends of red rubber feeding tubes can be cut to accommodate a 3-way stopcock; attaching these tubes to a continuous suction device is generally not recommended due to their tendency to collapse.
Guidelines for estimating chest tube size.
|
Cats & Dogs |
< 7 kg |
14-16 Fr |
|
Dogs |
7-15 kg |
18-22 Fr |
|
Dogs |
16-30 kg |
22-28 Fr |
|
Dogs |
> 30 kg |
28-36 Fr |
Clip and prepare the lateral thorax for aseptic surgery. In order to allow sufficient drainage, place additional holes in the tube by bending the tube and removing a notch with a pair of sterile scissor. Holes should not be greater than one-third the circumference of the tube. If using a commercial tube with a radiopaque line, place the last hole through the line in order to allow identification of its position on a thoracic radiograph. Make a small skin incision in the dorsal one-third of the lateral thoracic wall at the level of the 10th or 11th intercostal space. Advance the tube subcutaneously in a cranioventral direction for 3 to 4 intercostal spaces and introduce the tube through the muscle and pleura using the stylet or a large hemostat. When using a trocar tube, firmly grasp the tube 2 to 4 cm from the body wall with one hand while using the other hand to "pop" the tube through the intercostal musculature and pleura. This will prevent the tube from being inadvertently pushed further into the thorax than anticipated and damaging the lung or other thoracic structures. Feed the tube in a cranioventral direction to a predetermined point and before completely removing the trocar, clamp the tube with a hemostat. Place a purse-string suture in the skin around the tube (do not enter the lumen of the tube) and leave both ends of the suture long. Use this suture to perform a "Chinese-finger trap" or "Roman-sandal" suture. Connect the chest tube to a 3-way stopcock in order to increase the ease of thoracic drainage. Use a five-in-one (Christmas tree) adaptor or a female Luer lock (with small tubes) between the tube and the 3-way stopcock to ensure an air-tight seal. Use suture to secure the tube to the connecting devices so that they will not become inadvertently dislodged, resulting in a pneumothorax. For added safety when the chest cavity is not being suctioned, clamp the tube where it exits the body wall with a hemostat or C-clamp. Verify appropriate placement of the chest drain radiographically, prior to covering it with a loose bandage.
Drainage may be either intermittent or continuous. Generally, intermittent pleural drainage is adequate; however, in some situations (i.e., spontaneous pneumothorax, pleurodesis) continuous suction is preferable. Heimlich valves should only be used in medium to large dogs, since small dogs and cats may not develop sufficient expiratory pressure for effective drainage. Additionally, these valves are prone to malfunction if fluid is aspirated into the apparatus. "Milking" or "stripping" of chest tubes to prevent obstruction of the tube by clots has been recommended in the veterinary literature; however, these techniques generate high intrapleural pressures and may cause pulmonary damage.
Chest Tube Removal
With pleural effusion, remove the tube when the drainage decreases to a volume that is consistent with that caused by the presence of the tube itself (i.e., 2.2 ml/kg b.w./day). The tube can be removed in patients with pneumothorax once negative pressure has been achieved for 12 to 24 hours. Culture the end of the tube following removal if the tube has been present for several days, or if the animal shows signs of infection. Suture the skin incision with 1 or 2 simple interrupted sutures.
Continuous Thoracic Suction
If fluid accumulation is so rapid that intermittent drainage is not practical, or if adherence of the visceral pleura to the body wall is desired, continuous suction may be used. Two and three bottle systems and commercial suction units are available for veterinary use and are economical and simple to use (Figure 1). A continuous 10 to 15 cm negative pressure on the thorax effectively aspirates pneumothorax, increasing the likelihood of spontaneous sealing of large pulmonary defects. Slightly greater pressures may be necessary (up to 20 cm water) when viscous fluid is being drained.
| Figure 1. | 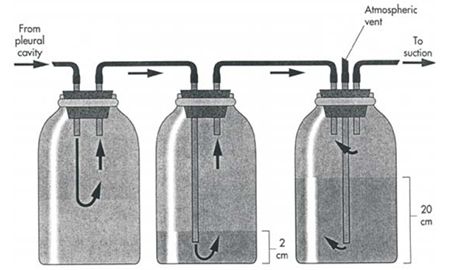
From: Fossum, TW: Small Animal Surgery, Mosby Publishing Co., St. Louis, Mo, 1997. |
|
| |
Connect the chest tube to a bottle that serves as an underwater seal (filled with 2 to 3 cm of sterile water) which in turn is connected to a suction bottle (also partially filled with water) attached to a suction device. Vary the amount of suction by raising or lowering the level of water in the suction bottle. A rigid plastic vent tube opened to room air serves to allow air to be aspirated into the bottle as the vacuum is applied. A third bottle interposed between the chest tube and the underwater seal bottle serves to collect fluid and prevent the level from rising in the underwater seal bottle as fluid is drained from the chest. This bottle is unnecessary in animals with pneumothorax. Alternately, a commercial continuous suction device may be used.