Abstract
Tilapia (Oreochromis niloticus) are a valuable and widely used fish, both as a food source and
as a biologic model in the scientific community.2 Culture of tilapia is a lucrative business world-wide, with
farms producing over 1,000,000 pounds of fish annually. Problems of infection with bacterial pathogens like Vibrio
spp. and Streptococcus spp.4 often wipe out tilapia farms as well as spread infection to the human
caretakers. Because of the growing concern about antibiotic residues in food for human consumption, as well as the increase
in antibiotic resistant microorganisms, vaccination has gained notoriety as a safe, inexpensive and effective means to
protect animals from infectious diseases. DNA vaccination is the most recent addition to the arsenal of vaccines. It
consists of plasmid DNA containing sequences encoding a protein of a pathogen. This plasmid is taken up by host cells, where
the encoded proteins are made and presented, initiating an immune response. Nucleic acid vaccines have elicited protective
immune responses against a variety of pathogens, including bacteria1 and parasites,3 in many different
vertebrate species. In order to evaluate the humoral immune response in tilapia to such an inoculation, a DNA vaccine was
produced using a bacterial β-galactosidase reporter gene expression plasmid. The reporter gene system allows expression
of a protein, that is foreign to the host but is non-pathogenic, which initiates an immune response. In the study, thirty
purebred O. niloticus were first grouped according to weight, so a similar number of fish at the same weight range
were in each group. Then the fish were randomly allocated into three groups of ten fish each. Group 1 fish each received 50
µg of the test plasmid, pCMVβ, containing the β-galactosidase gene. Group 2 fish each were immunized with 50
µof the empty, control plasmid, pCI, that lacks the reporter gene. Group 3 fish each received 100µl sterile saline
as a diluent control. Each treatment was administered by intramuscular injection. Blood was collected prior to the
immunization and then every other week for a total of five blood samples for each fish. The samples were screened for
β-galactosidase antibodies using indirect enzyme-linked immunosorbent assay (ELISA) with an anti-tilapia IgG secondary
antibody and an anti-rabbit antibody conjugated with horseradish peroxidase (HRP). Each sample was analyzed in duplicate.
Tilapia anti-β-galactosidase antibody titers were calculated as the reciprocal of the highest dilution that was two
standard deviations above the average optic density of the conjugate controls. Geometric mean anti-β -galactosidase
antibody titers were calculated at 0, 14, 28, 42, and 54 days post initial inoculation (Fig. 1). Analysis of variance
(ANOVA) of log-transformed anti-ß-galactosidase antibody titers was used to evaluate the differences in antibody titers
among the three experimental groups. Unfortunately, no significant difference was found among the groups, P = 0.6945.
The highest ELISA antibody titer achieved was 1:6400. This occurred in all study groups, even in pre-bleeds of saline
control fish. Both the saline control and empty plasmid groups produced varying β-galactosidase antibody titers from
week to week. Thus, the DNA vaccination with this reporter gene system did not elicit a consistent humoral immune response
in tilapia. Because some fish in all three groups had high levels of β-galactosidase antibodies prior to inoculation
with the three treatments, there is a possibility that tilapia encounter the bacterial β-galactosidase from their
normal flora creating circulating anti-β-galactosidase antibodies. Immunization with a different reporter gene, that is
novel to tilapia, may result in more encouraging data.
| Figure 1. | 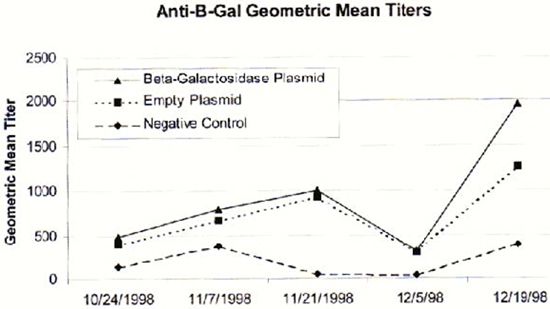
Geometric mean anti-β-galactosidase antibody titers of saline control
(100µ, i.m.), empty plasmid control (50µ, i.m.) and test plasmid tilapia treatments (50µ, i.m.). Tilapia, Oreochromis niloticus, were inoculated with treatments on 24 October 1998. |
|
| |
References
1. Bonato VL, VM Lima, RE Tascon, DB Lowrie, CL Silva. 1998. Identification and
characterization of protective T cells in hsp65 DNA vaccinated and Mycobacterium tuberculosis-infected mice.
Infection and Immunity 66:169-175.
2. de la Fuente J, I Guillen, R Martinez, MP Estrada. 1999. Growth regulations and enhancement
in tilapia: basic research findings and their applications. Genetic Analysis 15:85-90.
3. Waine GJ, W Yang, J C. Scott, DP McManus, BH Kalinna. 1997. DNA-based vaccination using
Schistosoma japonicum (Asian blood-fluke) genes. Vaccine 15:846-848.
4. Weinstein MR, M Lilt, DA Kertesz, P Wyper, D Rose, M Coulter, A McGeer, R Facklam, C
Ostach, BM Willey, A Borczyk, DE Low. 1997. Invasive infections due to fish pathogen Streptococcus iniae. S. iniae
Study Group. New England J. Med. 337:589-94.