Clarke E. Atkins, DVM, DACVIM (Internal Medicine & Cardiology)
Department of Clinical Sciences, North Carolina State University, College of Veterinary Medicine
Therapeutic Advances in the Management of Heart Disease: An Overview
The management of heart failure can be logically divided by the specific disease, its severity, and the type of signs present. Below is a figure outlining the use of drug classes for heart failure, according to severity (NYHA classification) and a table with indications and dosages.
Although controlled exercise has proved beneficial in human cardiac disease, some exercise restriction is logical in all forms of heart failure and can be progressively curtailed as the disease progresses. Since sodium retention is a major contributor to congestion, dietary NaCl restriction has long been used in the management of heart failure. Recently, it has become clear that extreme sodium restriction actually activates the renin-angiotensin aldosterone system (RAAS) and may contribute to renal dysfunction, particularly when ACE-I are used. Also it tends to make diets unpalatable. For these reasons, I now recommend only moderate salt restriction (e.g., senior, "early cardiac" or renal diets); although, terminally, more extreme NaCl restriction (e.g., a cardiac diet) may be necessary. Exercise and sodium restriction are instituted in NYHA phase II.
Diuretic therapy has long been the cornerstone in the management of congestive signs. We now know that extensive diuresis also activates the RAAS system. For this reason, I do not recommend diuresis as monotherapy and the dosage should be minimized to avoid RAAS activation, dehydration, azotemia, and hypokalemia. With angiotensin converting-enzyme inhibitors (ACE-I), the diuretic dosage can typically be reduced by 50%. Terminally, increasing levels of diuresis may, however, be necessary. Furosemide (Lasix) is by far the most commonly used diuretic. It is the drug of choice for emergency management of pulmonary edema. In refractory cases and in cases complicated by hypokalemia, potassium-sparing diuretics may provide an additive effect. Diuretics are employed in late phase II or III. The old diuretic, spironolactone, has found new promise in heart failure, as an aldosterone receptor blocker. In the event of refractory heart failure, continuous rate IV infusion of lasix has shown to be superior to IV bolus for the first 8 hours and for chronic refractoriness, there is promise that torsemide may be of use.
Conventional vasodilator therapy has been largely replaced with the advent of ACE-I. Nevertheless, nitroglycerin is useful in emergency situations to reduce preload and pulmonary edema and may be used chronically in refractory cases, as well. Hydralazine can be used to rescue dogs failing despite polypharmacy, to reduce cough due to severe mitral regurgitation, and possibly, to reduce pulmonary hypertension in heart failure due to HWD or other causes. In general, conventional vasodilators are employed in late phase III or phase IV.
ACE-I have become a cornerstone in the chronic management of heart failure and may be employed early (phase II) for reasons outlined above. There is evidence that they slow progression of heart failure in people and animals and they prolong life, improve quality of life, reduce electrolyte abnormalities, and blunt pathological remodeling. As suggested above, diuretic doses can and should be reduced in the presence of concomitant ACE-inhibition. In the case of enalapril (Enacard), the dose can be doubled in refractory cases.
New agents (or new uses for older agents) have targeted the neurohormonal abnormalities attendant to heart failure. These include beta blockers, such as carvedilol or metoprolol to blunt the sympathetic nervous system in heart failure; neutral endopeptidase inhibitors (e.g., ecadotril) which interfere with the breakdown of ANP, a hormone which has many effects opposite that of the RAAS; and angiotensin II receptor blockers (e.g., losartan) which have similar effects as ACE-I., but by blocking receptors, rather than formation of angiotensin II. Antioxidants and anti-cytokine therapies will see use in the future as well.
Beta-Blockers, such as metoprolol and carvedilol have earned a place in the management of heart failure in human dilated cardiomyopathy. Their rationale is derived from the large body of evidence as to the harmful nature of the sympathetic nervous system (SNS) in the syndrome of CHF. Their use has been slowly accepted because of the negative inotropic effect and difficulties in titrating to an effective dose. Nevertheless, improved quality of life, exercise tolerance, and survival have all been experienced in multiple clinical trials with carvedilol and metoprolol. Carvedilol, a non-selective beta- and alpha-blocker also has oxygen radical scavenging capabilities and reduces endothelin release. Hence the drug, in addition to sparing the heart the effects of the SNS, is a vasodilator and antioxidant, reduces heart rate, and has antiarrhythmic properties. Carvedilol has 2 major drawbacks. First it is a negative inotrope so is difficult to use with severely symptomatic patients. Secondly, it is expensive. The first drawback is overcome by starting early in the disease process, avoiding its use in NYHA phase IV, and beginning at a very low dosage, titrating toward a target dose of 25 mg BID in a large breed dog. At NCSU, a Doberman pinscher would be started at 3.125 (or even 1.56) mg QD for 2 weeks, then BID x 2 weeks, then 6.25/3.125 mg for 2 weeks, etc, until a full dose of 25-50 mg daily, divided BID, is achieved or the patient shows signs of intolerance. If intolerance develops (usually lassitude, inappetance, and hypotension), the dosage is dropped to the last tolerated dosage for 2-4 weeks and then an attempt is made to increase as previously described. If the patient cannot tolerate increases in carvedilol dosage, the last tolerated dosage is accepted as maximum. Human studies indicate that, while the benefit is lessened, sub-optimal dosages still provide benefits. The second drawback--cost--is overcome, if necessary, by using atenolol, a much less expensive, selective B1 receptor blocker. The compromise is the lack of vasodilatory and antioxidant properties and an inconvenient formulation for the early titration period. The dosage and titration schedule for atenolol approximates that of carvedilol, although fragmenting 25 mg tablets to 3.125 mg is challenging.
There are not data on beta-blockers in naturally-acquired canine mitral regurgitation, though there are data in experimental models, indicating hemodynamic and remodeling benefit, using high doses of atenolol. Additionally, there are clear data indicating quality of life and survival benefit in humans with CHF, treated with beta-blockers. Unfortunately, in addition to the cost disadvantage, dosing these agents is somewhat difficult in small dogs and requires special formulation. The currently recommended dosage for carvedilol is 0.25 mg/kg BID, increasing every 2 weeks until a target of 0.8-1.0 mg/kg BID is achieved.
Inotropic therapy, other than digoxin, has fallen largely into disfavor because they do not prolong life, may worsen arrhythmias and/or increase heart rate, can be given only intravenously, and newer therapies have replaced them. Dobutamine, dopamine, and amrinone can be used intravenously in cases of myocardial failure to rescue phase IV dogs. Digoxin, on the other hand, has enjoyed increased popularity because it is the only positive (although only weakly so) inotrope that is orally available, slows heart rate, and normalizes baroreceptor function. In addition, the RADIANCE and DIG trials in humans showed patients in heart failure denied digoxin had worsening of signs, quality of life, exercise tolerance and hemodynamic status. Digoxin is exquisitely indicated in heart failure with myocardial failure and, especially, if accompanied by SVT. A sound theoretical argument for digoxin can be made in dogs with heart failure, normal sinus rhythm and maintained myocardial function, however discretion must be used in these dogs as other drugs will control signs with less danger of toxicity. Therefore in some dogs (e.g., 4 pound Pomeranian with mitral regurgitation), the owner, inherent appetite, renal function, and severity of signs must be considered. In general, digoxin should be instituted in late phase II with myocardial failure and phase III (at same time as diuretic) in dogs without myocardial failure.
Pimobendan, a newer phosphodiesterase 3 inhibiting inotrope, better termed an inodilator (inotrope and mixed vasodilator), has shown significant promise in the management of dilated cardiomyopathy and probably mitral regurgitation. This drug, currently being used widely at 0.2-0.6 mg/kg divided BID (given 1 hour before feeding) in Europe and Australia, is thought to work, in part, by sensitizing the troponin C complex to calcium. The aforementioned association between postive inotropes and sudden death has not been recognized with pimobendan, reputedly because there is less or no increase in intracellular calcium and because of its arteriolar dilating capacity, which unloads the ventricles. A prospective study by Fuentes, et al. demonstrated improved survival in Doberman pinschers with dilated cardiomyopathy and the PiTCH study prospectively showed improved clinical outcomes in dogs with DCM and mitral regurgitation, as compared with ACE-Inhibition. A retrospective study, comparing pimobendan to historical controls treated standardly, by Gordon and colleagues in dogs with mitral regurgitation showed improved survival, vertebral heart score, heart and respiratory rate, and left atrial size, without evidence of arrhythmogenesis.
Miscellaneous agents, such as oxygen, morphine, dobutamine, dopamine, epinephrine, calcium chloride, etc., have special uses in severe heart failure (phase IV) and/or CPR. Taurine and carnitine are nutritional additives that respectively have been advocated for heart failure in cats and dogs. Taurine has all but eliminated feline dilated cardiomyopathy. The jury is still out regarding the overall utility and specific indications for carnitine in dogs. Cocker spaniels with dilated cardiomyopathy have responded to taurine plus carnitine supplementation. Fish oils may improve appetite and blunt cardiac cachexia and Coenzyme Q10 has its advocates in the management of heart failure dilated cardiomyopathy. I reserve bronchodilators for dogs with known (or suspected) lower airway collapse or other respiratory disease, but these agents do not represent part of my routine protocol for the management of heart failure.
Lastly, some centers now employ new surgical procedures or interventions, such as mitral valve reconstruction, balloon valvuloplasty of the pulmonic and aortic valves, and percutaneous balloon or thorascopic pericardiotomy for the treatment heart failure.
| Figure 1. | 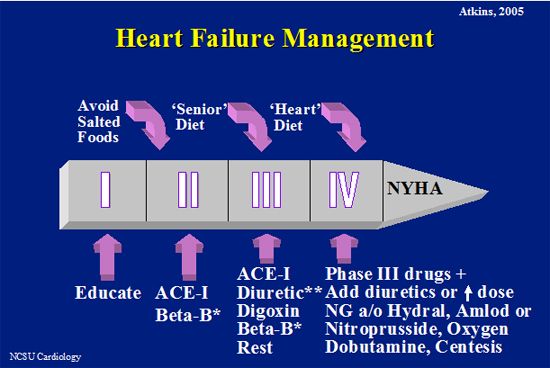
Overview of management of heart failure.
* Beta blockers are more easily introduced earlier in heart disease than later and in MR than DCM after symptoms are observed.
**Furosemide, spironolactone, or both.
NG=nitroglycerin,
Hydral = hydralazine,
Amlod = amlodipine.
Pimobendan may be added in Phase III or IV. Additionally, antiarrhythmic agents are added when needed and digoxin is instituted prior to heart failure in atrial fibrillation. |
|
| |
Medical management of factors contributing to signs of systolic heart failure in dogs.
|
Factor |
Strategy |
Agent and dosage |
|
Fluid retention/ Excessive Preload |
Salt restriction |
Senior diet, renal diet or, late in course, heart (heavily salt-restricted) diet |
|
Diuresis |
Furosemide 1 to 4 mg/kg s.i.d.-t.i.d. IV,IM,SC or PO or CRI at 0.66 mg/kg/min
Torsemide 0.2 mg/kg PO s.i.d.-t.i.d.
Hydrochlorothiazide or Aldactazide 2 to 4 mg/kg q.o.d.-b.i.d. PO
Chlorthiazide 20 to 40 mg.kg b.i.d. PO
Spironolactone 0.5-2.5 mg/kg b.i.d. PO |
|
Venodilation |
Triamterene 2 to 4 mg/kg/day PO
Nitroglycerin 2% ointment 1/4 in/5kg t.i.d. topically for 1st 24 hours
Captopril 0.5 to 2 mg/kg t.i.d. PO
Enalapril 0.5 mg/kg s.i.d.-b.i.d. PO
Benazepril 0.25-.5 mg/kg s.i.d. PO
Prazosin 1 mg t.i.d. if <15 kg; 2 mg t.i.d. if >15 kg
Sodium nitroprusside 1 to 5 μg/kg/min IV |
|
Neurohormonal Aberration |
Blunt RAAS |
Captopril 0.5 to 2 mg/kg t.i.d. PO
Enalapril 0.5-1 mg/kg s.i.d.-b.i.d. PO
Benazepril 0.25-.5 mg/kg s.i.d. PO
Spironolactone 0.5 mg/kg/day PO
Angiotensin II receptor blocker (e.g., Losartan) dosage TBD |
|
Blunt SNS |
Digoxin 0.005 to 0.01 mg/kg or 0.22 mg/m2 body surface b.i.d. PO for maintenance.
Propranolol 5 to 40 mg t.i.d. PO
Atenolol 0.25-1 mg/kg PO1
Carvedilol 0.1-.2 mg/kg s.i.d PO, increasing to .5-1mg/kg b.i.d. over 6 wks |
|
Increased afterload |
Arterial vasodilation |
Hydralazine 1 to 3 mg/kg b.i.d. PO
Captopril 0.5 to 2 mg/kg t.i.d. PO
Enalapril 0.5 mg/kg s.i.d.-b.i.d. PO
Benazepril 0.25-.5 mg/kg s.i.d. PO
Prazosin 1 mg t.i.d. PO if <15 kg; 2 mg t.i.d. if >15 kg PO
Sodium nitroprusside 1 to 5 μg/kg/min IV
Diltiazem 0.1-0.2 mg/kg IV slowly; 0.5-1.5 mg/kg t.i.d. PO
Amlodipine 0.1-0.2 mg/kg s.i.d.-b.i.d. PO
Sildenafil 0.5-1 mg/kg s.i.d.-b.i.d. PO |
|
Diminished contractility* |
Positive inotropic support |
Digoxin 0.005 to 0.01 mg/kg or 0.22 mg/m2 body surface b.i.d. PO for maintenance. Rapid oral: 0.01 mg/kg b.i.d. to 0.02 mg/kg t.i.d. for 1 day, then to maintenance.
Rapid IV: 001 to 0.02 mg/kg given one half IV immediately and one fourth IV at 30- to 60-minute intervals p.r.n.
Digitoxin 0.01 to 0.03 mg/kg t.i.d. PO. Rapid IV:0.01 to 0.03 mg/kg, given one half IV immediately and one fourth IV at 30- to 60-minute intervals p.r.n.
Dobutamine 1.5 to 20 μg/kg/min IV for <72 hours
Dopamine 2 to 10 μg/kg/min IV for <72 hours
Amrinone 1 to 3 mg/kg IV followed by 10 to 100 μg/kg/min
Pimobendan 0.25 mg/kg b.i.d. PO |
|
Abnormal HR:
Bradyarrhythmia |
Normalize HR, rhythm |
Atropine sulfate 0.1 to 0.2 mg/kg SC or IM
Glycopyrrolate 0.05 to 0.01 mg/kg SC or IM
Dopamine 2 to 10 μg/kg/min IV for <72 hours
Terbutaline 1.25 to 2.5 mg b.i.d.-t.i.d. PO
Pacemaker implantation |
|
Supraventricular tachycardia |
|
Digoxin: Same as above
Esmolol 100-500 μg/kg IV rapidly; Atenolol 6.25-25 mg PO s.i.d.-b.i.d.1
Propranolol 5 to 40 mg t.i.d. PO; 0.1 to 0.3 mg/kg IV slowly
Verapamil1 1 to 5 mg/kg t.i.d. PO; 0.05 to 0.25 mg/kg IV slowly
Diltiazem1 0.5-1.5 mg/kg t.i.d. PO; 0.1-.25 mg/kg over 2 min IV |
|
Ventricular tachycardia |
|
Lidocaine 2 to 4 mg/kg IV; repeat up to 8 mg/kg over 20 minutes
Procainamide 5 to 15 mg/kg t.i.d.-q.i.d. PO; 5 to 10 mg/kg IV
Quinidine 5 to 15 mg/kg q.i.d. PO
Esmolol, Propranolol, Atenolol: Same as above
Tocainide 5 to 10 mg/kg t.i.d. PO
Sotalol 1-2 mg/kg b.i.d.
Amiodarone 10-20 mg/kg s.i.d. x 7-10 days, then maintain at 5 mg/kg q48h to 15mg/kg s.i.d. |
Adapted from Atkins, CE: Atrioventricular Valvular Insufficiency in Allen DG (ed): Small Animal Medicine, Lippincott, 1992
*In most instances of mitral insufficiency, positive inotropic support is unnecessary.
1Calcium channel (verapamil and diltiazem) and Beta blockers (propranolol, esmolol, atenolol) should be used with caution in patients in heart failure.
s.i.d. = once daily; b.i.d. = twice daily; t.i.d. = three times daily; q.i.d. = four times daily; IM = intramuscularly; IV = intravenously; SC = subcutaneously; PO = per os; prn = as needed.
Management of Heart Failure: Neurohumoral Modulation, Diuretics, and Salt Restriction
Our understanding of the pathogenesis and management of heart failure has markedly changed over the last 20 years. During this time we have learned that the heart may fail due to diastolic dysfunction, as well as systolic dysfunction; that hemodynamic alterations and their management are less important than the body's own maladaptive neurohormonal response to a fall in cardiac output; that drugs which improve hemodynamics may actually result in long-term harm; and that the greatest clinical benefits result from therapies which blunt the body's neurohormonal response in heart failure. In addition, there have been a plethora of new procedures, drugs, and even drug classes introduced for the management of cardiac disease.
Some of the most important clinical ramifications of heart failure, such as dyspnea (due to pulmonary edema or pleural effusion) and ascites, are directly attributable to sodium and fluid retention resulting from activation of the renin-angiotensin-aldosterone system (RAAS). Management of the signs of congestive heart failure (CHF) has relied upon the use of natriuretic diuretics (furosemide), restriction of dietary sodium, and more recently angiotensin converting-enzyme inhibitors (ACE-I) which, by blocking aldosterone production, combat sodium retention and congestion. In addition, as vasodilators, ACE-I unload the heart, improving cardiac output and exercise, normalize electrolyte aberrations, and blunt the pathological cardiovascular remodeling produced by angiotensin II and aldosterone.
While off-loading therapy with the aforementioned drug groups can be life-saving, their use can be associated with adverse side-effects. Most notable of these are hypotension, azotemia, renal failure, and arrhythmias. Certain complications are more apt to occur when combinations of drugs are used. Because of the potential for such side effects, these drugs are best employed in specific sequence and combinations. The following discussion relates to their use in the management of chronic heart failure.
Angiotensin Converting-Enzyme Inhibitors
In landmark veterinary studies of enalapril in NYHA phase III and IV heart disease (moderate to severe heart failure), due to mitral regurgitation (MR) and dilated cardiomyopathy (DCM), enalapril improved survival by >100% as well as reducing pulmonary edema and, improving quality of life scores.1-3 Exercise capacity is also improved in dogs with experimental mitral insufficiency.4 Benazepril has likewise been shown to improve survival.5 ACE-I have proven to provide additional benefits in human patients by blocking pathological remodeling, presumably slowing progression of heart disease and by normalizing serum electrolyte concentrations.Today, ACE-I represent the cornerstone in the chronic management of CHF. They are indicated in virtually all cases of systolic heart failure in which they are tolerated.
There was early concern regarding the renal safety of these compounds6-8 and all ACE-I, which have enjoyed extensive clinical use, have been associated with renal dysfunction, usually temporary.9 There has been speculation that, at very high doses (180x the clinical dosage), ACE-I have direct nephrotoxic effects but it is generally felt that the major impact of ACE-I on the kidney, with clinically relevant dosages, is through production of hypotension, with reduced renal perfusion pressure and resulting in worsening of azotemia.10 To date, veterinary clinicians have had experience with enalapril, captopril, benazepril, and lisinopril. Of these, only enalapril has been extensively studied and is licensed for use in management of heart failure in the United States, though benazepril has been marketed in Europe and Canada. The active metabolite of benazepril is reportedly excreted both in the bile and in the urine so that lower serum concentrations are evident in experimental renal disease.11 The clinical relevance of this is unclear. Over 10 years of veterinary clinical experience with ACE-I (mainly captopril and enalapril) have taught us that their impact on kidney function is minimal even in the face of severe heart failure. When azotemia is observed, ACE-I are almost always being used in conjunction with diuretics and sodium restriction and hypotension results. Typically, cessation of diuretic therapy or reduction in the dosage results in the reversal of azotemia.9
In studies of enalapril in NYHA phase III and IV heart disease (moderate to severe heart failure), due to MR and DCM, there was actually a lower incidence of azotemia in the enalapril-treated group than the placebo-treated group.1-3,12 Furthermore, in a study of enalapril's role in the delay or prevention of heart failure due to naturally-occurring MR, showed that enalapril at the standard dosage of 0.5 mg/kg daily had no effect on serum creatinine concentrations, as compared to placebo.13
In fact, evidence is building to prove benefit when ACE-I are administered chronically to both human and veterinary patients with naturally-occurring and experimental renal failure.14-20 Mechanisms for this improvement are postulated to be the antihypertensive effect, reduction of angiotensin II-induced mesangial cell proliferation, and renal vasodilatory effects of ACE-I, the latter related to a fall in renal filtration pressure and proteinuria.14-16 Enalapril has recently been shown to reduce urine protein loss and reduce blood pressure in naturally-occurring canine glomerulonephritis.18 Likewise, benazepril reduced azotemia and proteinuria in a short-term study of experimental and naturally-occurring renal insufficiency in cats19 and lowered BUN and creatinine concentrations and blood pressure in cats with polycystic kidney disease.20
As mentioned above, ACE-I have the potential to produce symptomatic hypotension. This is due to the mixed vasodilatory effect of this group of drugs and is typically observed when ACE-I are used in conjunction with other off-loading therapies, such as vasodilators, diuretics, and sodium restriction. Hypotension is reversed by altering drug therapies but may be problematic in producing azotemia, inappetance, weakness, lassitude, and precipitating digitalis intoxication by reducing renal elimination.
Beta-Blockers
Beta-blockers, such as metoprolol and carvedilol have earned a place in the management of heart failure in human dilated cardiomyopathy.20a Their rationale is derived from the large body of evidence as to the harmful nature of the sympathetic nervous system (SNS) in the syndrome of CHF. Their use has been slowly accepted because of the negative inotropic effect and difficulties in titrating to an effective dose. Nevertheless, improved quality of life, exercise tolerance, and survival have all been experienced in multiple clinical trials with carvedilol and metoprolol. Carvedilol, a non-selective beta- and alpha-blocker also has oxygen radical scavenging capabilities and reduces endothelin release. Hence the drug, in addition to sparing the heart the effects of the SNS, is a vasodilator and antioxidant, reduces heart rate, and has antiarrhythmic properties.20b Carvedilol has 2 major drawbacks. First it is a negative inotrope so is difficult to use with severely symptomatic patients. Secondly, it is expensive. The first drawback is overcome by starting early in the disease process, avoiding its use in NYHA phase IV, and beginning at a very low dosage, titrating toward a target dose of 25 mg BID in a large breed dog. At NCSU, a Doberman pinscher would be started at 3.125 (or even 1.56) mg QD for 2 weeks, then BID x 2 weeks, then 6.25/3.125 mg for 2 weeks, etc, until a full dose of 25-50 mg daily, divided BID, is achieved or the patient shows signs of intolerance. If intolerance develops (usually lassitude, inappetance, and hypotension), the dosage is dropped to the last tolerated dosage for 2-4 weeks and then an attempt is made to increase as previously described. If the patient cannot tolerate increases in carvedilol, the last tolerated dosage is accepted as maximum. Human studies indicate that, while the benefit is lessened, sub-optimal dosages still provide benefits. The second drawback--cost--is overcome, if necessary, by using atenolol, a much less expensive, selective B1 receptor blocker. The compromise is the lack of vasodilatory and antioxidant properties and an inconvenient formulation for the early titration period. The dosage and titration schedule for atenolol approximates that of carvedilol, although fragmenting 25 mg tablets to 3.125 mg is challenging.
There are not data on beta-blockers in naturally-acquired canine mitral regurgitation, though there are data in experimental models, indicating hemodynamic and remodeling benefit, using very high doses of atenolol.20c Additionally, there are clear data indicating quality of life and survival benefit in humans with CHF, treated with beta-blockers. Unfortunately, dosing these agents is somewhat difficult in small dogs and this author has yet to routinely embrace this group of agents (carvedilol, atenolol, and metoprolol) in this setting, either before or after the onset of CHF.
Aldosterone Receptor Blockers
Spironolactone and eplerinone, aldosterone receptor blockers, used in the treatment of heart failure in humans, are thought to be effective by blocking the remodeling effects of aldosterone. It has been shown in people, but not dogs, that aldosterone and angiotensin II "escape" from ACE-inhibitor suppression weeks to months after institution of therapy.20d Spironolactone has been embraced by veterinary cardiologists for the treatment of CHF caused by dilated cardiomyopathy, mitral regurgitation, etc in dogs. As yet unpublished studies by Rausch and colleagues at NCSU demonstrated no risk for hyperkalemia in dogs treated concurrently with enalapril and spironolactone. The dosage used by this author for aldosterone receptor blockade is 0.5 mg/kg QD. The use of spironolactone as a diuretic is discussed below.
Sodium Restriction
The salt avidity that results from aldosterone secretion in heart failure has been well documented.21 Sodium restriction contributes to signs of congestion (pulmonary edema, ascites, pleural effusion) and hence reduction in salt intake is logical. There are little data on clinical outcomes with such strategy but stringent salt restriction with diuresis has been shown to reduce total body sodium stores while, paradoxically, blunting acute furosemide-induced diuresis.22 Roudebush demonstrated that neither moderate nor severe salt restriction alone caused azotemia in aged, normal dogs, but when furosemide (3.2 mg/kg b.i.d.) was coupled with severe (but not moderate) salt restriction, serum creatinine rose by 63%--more than twice as much as in dogs receiving a diet with a standard sodium content.23 Furthermore, both moderate and severe salt restriction activated the renin-angiotensin-aldosterone system (RAAS) and when furosemide was added to the regimen, there was nearly a 6000-fold increase in serum aldosterone concentration with severe salt restriction. Finally, it is well established that salt restriction increases the likelihood of azotemia with ACE-I therapy.10
One can conclude that sodium restriction, while logical and likely useful in reducing total body sodium concentration and diuretic requirements, is not without a toll. This toll represents the tendency to increase azotemia with concurrent diuretic and ACE-I therapy and to activate the RAAS. Use of moderate salt restriction (e.g., a diet designed for renal patients with .22% sodium by dry weight) early in heart failure is advisable, with severe salt restriction (.10% sodium by dry weight) being reserved for patients refractory to therapy. Concurrent diuresis should be avoided as long as possible and ACE-I should accompany sodium restriction and diuretic therapy.
Diuretics
The most widely used diuretic is furosemide, a loop diuretic. It is potent and, while life-saving, it has the potential to produce azotemia, hypotension, and electrolyte disturbances, to lower cardiac output and to activate the RAAS.23,24 Fatal arrhythmias have been associated with "non-potassium sparing diuretics".25 Furosemide is not primarily nephrotoxic, though it can potentiate other nephrotoxic drugs. It produces prerenal azotemia by dehydration and hypotension and has a synergistic effect in diminishing renal function when used with either ACE-I or sodium restriction.10,23,24
Furosemide is the drug of choice for life-threatening pulmonary edema. Otherwise, it should be used only as needed to control signs of congestion. In other words, because it activates the RAAS, lowers blood pressure and cardiac output, causes azotemia and electrolyte disturbances, and potentiates adverse effects of other cardiac therapies, it should not be used as a monotherapy (i.e., with the exception of emergency therapy, furosemide therapy should always be accompanied by an ACE-I) and should be used at the lowest dosage compatible with good quality of life. If azotemia develops in a patient receiving polypharmacy, the first change should be the decrement or cessation of furosemide.
The aldosterone antagonist, spironolactone, has received renewed interest with a report that survival was prolonged in humans with heart failure when spironolactone (~0.3 mg/kg QD) was administered concurrently with conventional therapy in NYHA phase IV patients.26 Because spironolactone is a weak diuretic, particularly at the modest dosage used in this study, the investigators concluded that benefits were due to blunting the adverse effects of aldosterone. This drug might logically be used early in heart failure for this reason, but there are no data for early or pre-heart failure states. As mentioned above, we have seen no increase in electrolyte abnormalities with concurrent ACE-inhibition and adlosterone blockade. It is meant to compensate for temporary or incomplete suppression of aldosterone secretion by ACE-I and should be used concurrently with ACE-I. It is also employed as an adjunctive diuretic at 1-2 mg/kg QD-BID with a loop diuretic, such as furosemide. Enhanced diuresis is enjoyed when 2 diuretics work synergistically in different parts of the nephron.
Conclusion
Of the therapeutic strategies discussed, loop-diuretic therapy has the greatest potential for adverse side-effects (hypotension, azotemia, activation of RAAS, electrolyte disturbances and fatal arrhythmias). Therefore, except in emergencies, furosemide should not be used as a monotherapy and should be used at the lowest dosage preventing signs of CHF. Salt restriction has similar, but lesser effects on RAAS activation, and potentiates diuretic- and ACE-I-induced tendencies toward azotemia.3,4 Therefore, moderate, rather than severe salt restriction, is indicated until signs of heart failure become refractory. Of the off-loading therapies under discussion, only ACE-I have been shown to benefit heart failure while blunting other pathophysiological processes (RAAS activation, electrolyte abnormalities, aldosterone- and angiotension II-induced cardiac remodeling, and renal dysfunction). Therefore, if either azotemia or hypotension is noted in a patient being managed for heart failure, the diuretic should first be discontinued or the dosage reduced, being reinstituted as necessary. Reduction or cessation of ACE-I is employed only if altering the diuretic dosage is ineffectual. Though ACE-I are generally safe, BUN and creatinine, as well as serum potassium concentration and systemic blood pressure should be monitored periodically, particularly if sodium restriction and/or diuretic therapy are utilized concurrently. Finally, when any of these agents are utilized, either alone or in combination, if caution is exercised and hypotension avoided, there is little risk of significant renal impairment.
Beta-blockers are indicated in DCM (NYHA Phase I, II, and III). Although theoretically indicated, this author does not employ this therapy routinely for this modality in MR. Aldosternone receptor blockers are useful in CHF, but their exact role is yet to be defined. I use spironolactone to aid in RAAS-suppression and to enhance diuresis in refractory CHF.
References
1. Improve Study Group. Acute and short-term hemodynamic, echocardiographic, and clinical effects of enalapril maleate in dogs with naturally occurring acquired heart failure: Results of invasive multicenter prospective veterinary evaluation of enalapril study. J Vet Intern Med 1995; 9:234-242.
2. Cove Study Group. Controlled clinical evaluation of enalapril in dogs with heart failure: Results of the cooperative veterinary enalapril study group. J Vet Intern Med 1995; 9:234-242.
3. LIVE Study Group. Effects of enalapril on survival in dogs naturally acquired heart disease: Results of long-term investigation of veterinary enalapril (LIVE) study group. J Amer Vet Med Assoc 1998; 213:1573-1577.
4. Hamlin RL, Benitz AM, Ericsson GF, et al.: Effects of enalapril on exercise tolerance and longevity in dogs with heart failure produced by iatrogenic mitral regurgitation. J Vet Intern Med 1996; 10:85-87.
5. Bench Study Group: The effect of benazepril on survival times and clinical signs of dogs with congestive heart failure: Results of a multicenter, prospective, randomized, double-blinded, placebo-controlled, long-term clinical trial. J Vet Cardiol 1999; 1:7-18.
6. Packer M, Leen WH, Medina N, et al. Functional renal insufficiency during long-term therapy with captopril and enalapril in severe, chronic, heart failure. Ann Intern Med 1987; 106:346-352.
7. Schlessinger DP, Rubin SI. Potential adverse effects of angiotensin-converting enzyme inhibitors in the treatment of congestive heart failure. Compend Cont Ed Pact Vet 1994; 16:275-283.
8. Roudebush P, Allen TA. The effect of combined therapy with captopril, furosemide, and a sodium-restricted diet on serum electrolyte concentrations and renal function in normal dogs and dogs with congestive heart failure. J Vet Intern Med 1994; 8:337-342.
9. Wynckel A, Ebikili B, Melin JP, et al. Long-term follow-up of acute renal failure caused by angiotensin converting enzyme inhibitors. Amer J Hypertens 1998; 11:1080-1187.
10. MacDonald JS, Bagdon AJ, Peter CP, et al. Renal effects of enalapril in dogs. Kidney International 1987; S20:148-153.
11. Lefebvre HP, Laroute V, Concordet D, Toutain P. Effects of renal impairment on the disposition of orally administered enalapril, benazepril, and their metabolites. J Vet Intern Med 1999; 13:21-27.
12. Merck AgVet. Enacard package insert. 1994.
13. Atkins CE, Brown WA, Coats JR, et al. Effects of long-term administration of enalapril on clinical indicators of renal function in dogs with compensated mitral regurgitation. J Am Vet Med Assoc. 2002; 221:654-658.
14. Abraham PA, Opsahl JA, Halstenson CE, et al. Efficacy and renal effects of enalapril therapy for hypertensive patients with chronic renal insufficiency. Arch Intern Med 1988; 148:2358-2362.
15. Praga M, Hernandez E, Montoyo C, et al. Long-term beneficial effects of angiotensin-converting enzyme inhibition in patients with nephrotic proteinuria. Amer J Kidney Dis 1992; 20:240-248.
16. Maschio G, Alberti D, Gerard J, et al. Effect of the angiotensin-converting enzyme-inhibitor benazepril on the progression of chronic renal failure. New Eng J Med 1996; 334:939-945.
17. Brown SA, Brown CA, Jacobs G, et al. Hemodynamic effects of angiotensin converting enzyme inhibition (benazepril) in cats (abst). J Vet Intern Med 1999; 13:250.
18. Grauer GF, Creco DS, Getzy DM, et al. Effects of enalapril versus placebo as a treatment for canine idiopathic glomerulonephritis. J Vet Intern Med. 2000; 14:526-533.
19. Watanabe T, Mishina M, Wakao Y. Studies of the ACE inhibitor benazepril in an experimental model and in clinical cases of renal insufficiency in cats (abst). J Vet Intern Med 1999; 13:252.
20. Miller RH, Lehmkuhl LB, Smeak DD, et al. Effect of enalapril on blood pressure, renal function, and the renin-angiotensin-aldosterone system in cats with autosomal dominant polycystic kidney disease. Amer J Vet Res 1999; 60:1516-1525.
20a. Bristow, MR, Gilbert, EM, Abraham, WT, et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. Circulation 1996; 94:2807-2816
20b. Suwanakiet, S, Miyamoto, M, Nakayama, T, Hamlin, RH. Acute cardiovascular effects of carvedilol in healthy dogs. Amer J Vet Res 2000; 61:57-60.
20c. Tsutsui, H., Spinale, FG, Nagutsu, M, et al. Effects of chronic B-adrenergic blockade on the left ventricular function and geometry because of chronic mitral regurgitation. J Clin Invest 1994; 93:2639-2648.
20d. Xiu JC, Wu P, Xu JP, et al. Effects of long-term enalapril and losartan therapy of heart failure on cardiovascular aldosterone. J Endocrinol Invest 2002; 25:463-468.
21. Barger AC, Muldowney FP, Liebowitz MR.. Role of the kidney in the pathogenesis of congestive heart failure. Circulation 1959; 20:273-281.
22. Wilcox SW, Mitch WE, Kelly RA, et al. Response of the kidney to furosemide: Effect of salt intake and renal compensation. J Lab & Clin Med 1983; 102:450-458.
23. Roudebush P, Allen TA: Effect of dietary sodium and furosemide on hematologic, biochemical, and endocrine parameters in normal geriatric dogs (abst). J Vet Intern Med 1996; 10:171.
24. Ikram H, Chan W, Espiner EA, Nicholls MG. Haemodynamic and hormone responses to acute and chronic frusemide therapy in congestive heart failure. Clin Sci and Molec Med 1980; 59:433-440.
25. Cooper HA, Dries DL, Davis CE. Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction. Circulation 1999; 100:1311-1315.
26. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. New Eng J Med 1999; 341:709-717.