Carmel T. Mooney, MVB, MPhil, PhD, DECVIM-CA, MRCVS
Department of Small Animal Clinical Studies, Faculty of Veterinary Medicine, University College Dublin, Belfield
Dublin, Ireland
Most diabetic dogs and cats are stabilised and managed without difficulty. However, many require investigation of poor glycaemic control at some point in their diabetic life. The most common and frustrating problem is the diabetic animal with persistent or recurrent clinical signs of hyperglycaemia despite administration of increasingly higher doses of insulin. This may be noted early on in diabetic stabilisation but can occur in any animal previously considered stable even after prolonged periods of time. These animals are at increased risk of developing the various complications associated with diabetes mellitus including acute pancreatitis, cataract formation and blindness, worsening neuropathies, increased susceptibility to infections and life threatening ketoacidosis. Most clinicians consider diabetic patients unstable when the injected insulin dose approaches or exceeds 2 IU/kg without adequate control of clinical signs.
Minimising the risk of unstable diabetics
In order to minimise both the number of patients and possible causes, it is important for clinicians to define their goals for glycaemic control and to preselect a 'practice regimen' that can achieve those goals.
The long-term complications of diabetes mellitus are not as severe in small animals as they are in humans nor is it as easy to administer multiple insulin injections based on simultaneous blood glucose measurement. An expectation that reference range blood glucose concentrations will be maintained throughout the day in dogs or cats with either once or twice daily injections is unrealistic. A more appropriate goal is to achieve good control of clinical signs with a reasonable dose of insulin using a regime easily adaptable to the owners' lifestyle and ability.
There are numerous protocols currently recommended for diabetic control, all of which differ with regard to the type of insulin, species of origin, timing of injections, feeding regime, owner and veterinary input and cost. Because each is associated with its own specific problems, it is wise for a practice to choose one or two regime best suited to their facilities and to use these routinely unless indicated otherwise. Overall, using a regime that culminates in a 'fixed dose' of insulin is preferable to one in which the owner frequently changes the insulin dose based on urine glucose measurements. Glucose concentrations take three to four days to equilibrate after each dose adjustment. Frequent and particularly large, dose adjustments can result in dramatic signs of diabetic instability and fluctuating requirements. In addition, if a small number of 'fixed dose' protocols are routinely used, the variety of different problems that may be encountered will be significantly reduced allowing for more successful diagnosis and intervention.
Recognition of unstable diabetics
The existence of a problem is usually indicated by
 Owner complaints of poor control of clinical signs and abnormal physical examination findings (particularly polyuria/polydipsia, polyphagia, lethargy, weight loss, deterioration in coat condition, worsening of hepatomegaly or peripheral neuropathies, development of cataracts) usually despite a previous increase in the dose of insulin administered
Owner complaints of poor control of clinical signs and abnormal physical examination findings (particularly polyuria/polydipsia, polyphagia, lethargy, weight loss, deterioration in coat condition, worsening of hepatomegaly or peripheral neuropathies, development of cataracts) usually despite a previous increase in the dose of insulin administered
 Inappropriately high nadir blood glucose concentration, high fasting blood glucose concentration or mean blood glucose concentration during an 8 hour period
Inappropriately high nadir blood glucose concentration, high fasting blood glucose concentration or mean blood glucose concentration during an 8 hour period
 Inappropriately high fructosamine concentration Fructosamine is a glycated protein, the concentration of which is dependent on the serum concentration and halflife of albumin (1-3 weeks) and the prevailing blood glucose concentration over that time. Generally, a serum fructosamine concentration greater than approximately 450 and 600 µmol/l indicates poor diabetic control in dogs and cats, respectively. However, results from individual laboratories differ. Glycosylated haemoglobin may be measured as an alternative. Its concentration is dependent on the half-life of haemoglobin, estimated to be between six and 14 weeks in dogs and cats.
Inappropriately high fructosamine concentration Fructosamine is a glycated protein, the concentration of which is dependent on the serum concentration and halflife of albumin (1-3 weeks) and the prevailing blood glucose concentration over that time. Generally, a serum fructosamine concentration greater than approximately 450 and 600 µmol/l indicates poor diabetic control in dogs and cats, respectively. However, results from individual laboratories differ. Glycosylated haemoglobin may be measured as an alternative. Its concentration is dependent on the half-life of haemoglobin, estimated to be between six and 14 weeks in dogs and cats.
Controversy surrounds the best method for assessing diabetic stability. There is no doubt that a single blood glucose measurement is the least useful as it can potentially be below or within the reference range at any time point in poorly controlled animals. Owner assessment and clinical signs are extremely valuable methods of monitoring glycaemic control(1). Fructosamine also provides valuable information but the long period of glucose control glycosylated haemoglobin assesses compromises its clinical usefulness.
Diagnosing potential causes
The potential causes can be broadly divided into four categories
 Problems with insulin administration and regime
Problems with insulin administration and regime
 Short duration of insulin activity
Short duration of insulin activity
 Somogyi overswing
Somogyi overswing
 Insulin resistance
Insulin resistance
Problems associated with insulin administration and regime may simply be identified by careful questioning of owners and by evaluating their technique. Problems can arise because the owner has not been sufficiently instructed or is not comfortable or experienced in the correct injection technique, the correct way of handling insulin or the necessity to adhere to the strict dietary/exercise regimen.
Specific problems with insulin can arise because of prior dilution and alteration of shelf-life, inactivation due to exposure to extreme heat, sunlight or vigorous shaking and use past its expiry date. Confusion can arise because of the interchangeable use of 100 IU/ml, 50 IU/0.5 ml and 40 IU/ml syringes and owners must be educated on the most appropriate syringe to use in their pet.
Diet and exercise also have considerable impact on glycaemic control in diabetic animals. Semi-moist diets high in soluble sugars should be avoided because their use is associated with persistently high insulin requirements. This apart, although specific diets are recommended to aid glycaemic control in both dogs and cats, it is more important that the diet fed is constant in calorific content, nutrient profile and volume and fed at the same time every day at a time coinciding with insulin injections. Significant under or overfeeding can lead to severe insulin resistance because of the development of weight loss and obesity, respectively. Exercise is also important and should be commensurate with the animal's ability, constant from day to day and timed to avoid peak insulin activity.
If problems are not identified after reviewing the above a full clinical examination is required. In some cases, the existence of a problem is obvious and further diagnostic tests can be carried out and appropriate action taken. However, in many cases the cause of the problem remains unclear and a serial blood glucose curve is indicated.
Serial blood glucose curves were once a routine part of the evaluation of the glycaemic control of diabetic dogs. However, recently they have fallen into disrepute because of the stress associated with their performance, particularly when animals are hospitalised, and the variability in results from day to day leading to opposite dosage recommendations from sequential curves in the same individual(5). However, in the investigation of unstable diabetics, serial blood glucose curves can provide information on the efficacy and duration of activity of insulin and are therefore an extremely valuable diagnostic tool in achieving the diagnosis in certain cases, or alternatively, in suggesting a shorter list of differentials which can be systematically evaluated.
If blood glucose curves are carried out under hospital conditions, it may be prudent to admit the animal for a day beforehand to reduce stress. The owners regime is strictly adhered to and blood glucose concentrations are measured every two hours over 12 hours for animals on twice daily insulin and as far as possible for 24 hours in animals on once daily dosing.
Blood glucose concentrations can be measured using standard laboratory techniques, point of care analysers or portable glucometers(3,7,8). There is some variability in the performance of individual glucometers and if used some comparison should be made with a laboratory reference technique. Blood glucose curves can also be carried out in the home environment using capillary blood obtained from the ear margin(2, 8). Continuous noninvasive reverse iontophoresis glucose measurement is currently under investigation in diabetic dogs and cats and may yet replace the need for blood glucose measurements.
Interpreting serial blood glucose curves
An example of a reasonable blood glucose curve presented in Figure 1.
 Short duration of insulin action: The duration of intermediate and long-acting preparations may be considerably less than 12 or 24 hours in individual animals (Figure 2). As a result hyperglycaemia is present for significantly prolonged periods each day and may occur as soon as 6 hours after insulin injection. Treatment involves changing the insulin type or frequency of injection. This is a frequent complication in cats treated with once daily intermediate-acting insulin.
Short duration of insulin action: The duration of intermediate and long-acting preparations may be considerably less than 12 or 24 hours in individual animals (Figure 2). As a result hyperglycaemia is present for significantly prolonged periods each day and may occur as soon as 6 hours after insulin injection. Treatment involves changing the insulin type or frequency of injection. This is a frequent complication in cats treated with once daily intermediate-acting insulin.
 Insulin-induced hyperglycaemia. The Somogyi overswing: This is a normal physiological response to hypoglycaemia that can give rise to problems in diabetic animals (Figure 3). Once blood glucose concentrations fall below normal, several physiological mechanisms are stimulated which increase blood glucose concentrations and minimise signs of hypoglycaemia. In diabetic animals, insulin cannot be released to 'dampen' this phenomenon. Treatment is achieved by decreasing the insulin dose by 20-30 %. Somogyi overswing is a common problem where insulin dose adjustments are made based on morning urine glycosuria. However, unrecognised short duration of insulin activity may be the inciting cause. Therefore, re-evaluation of a serial blood glucose curve may be necessary after the insulin dose is reduced. If hyperglycaemia is prolonged after Somogyi overswing, insulin resistance may be noted on a blood glucose curve.
Insulin-induced hyperglycaemia. The Somogyi overswing: This is a normal physiological response to hypoglycaemia that can give rise to problems in diabetic animals (Figure 3). Once blood glucose concentrations fall below normal, several physiological mechanisms are stimulated which increase blood glucose concentrations and minimise signs of hypoglycaemia. In diabetic animals, insulin cannot be released to 'dampen' this phenomenon. Treatment is achieved by decreasing the insulin dose by 20-30 %. Somogyi overswing is a common problem where insulin dose adjustments are made based on morning urine glycosuria. However, unrecognised short duration of insulin activity may be the inciting cause. Therefore, re-evaluation of a serial blood glucose curve may be necessary after the insulin dose is reduced. If hyperglycaemia is prolonged after Somogyi overswing, insulin resistance may be noted on a blood glucose curve.
 Insulin resistance: A diagnosis of insulin resistance is made by demonstrating persistent hyperglycaemia despite insulin administration (Figure 4). A range of possibilities exist (Table 1) and should be considered based on clinical findings and results of supportive diagnostic tests. The existence of concurrent disorders is most common(4,6).
Insulin resistance: A diagnosis of insulin resistance is made by demonstrating persistent hyperglycaemia despite insulin administration (Figure 4). A range of possibilities exist (Table 1) and should be considered based on clinical findings and results of supportive diagnostic tests. The existence of concurrent disorders is most common(4,6).
Summary
 Step 1: Gather information including the protocol used and the owners' ability to implement it. Check insulin and injection technique. Weigh and condition score the animal and then complete a full clinical examination.
Step 1: Gather information including the protocol used and the owners' ability to implement it. Check insulin and injection technique. Weigh and condition score the animal and then complete a full clinical examination.
 Step 2: Take action as indicated by step 1, e.g., replace diluted insulin, re-educate clients, perform diagnostic tests as indicated by clinical signs.
Step 2: Take action as indicated by step 1, e.g., replace diluted insulin, re-educate clients, perform diagnostic tests as indicated by clinical signs.
 Step 3: Perform a blood glucose curve.
Step 3: Perform a blood glucose curve.
 Step 4: Take action as indicated by step 3 (decrease insulin dose or switch to twice daily insulin injections). If insulin resistance has been identified consider potential concurrent disease in the light of the individual animal. Carry out routine screening laboratory analyses, proceeding with more investigative techniques depending on results. If a concurrent problem is not identified consider insulin antibodies or poor subcutaneous absorption. It is possible for animals to develop an immunological resistance to insulin preparations from species without identical insulin structure. Switching species of insulin will correct the problem. Administration of insulin subcutaneously does not necessarily imply absorption and poor subcutaneous absorption has been identified particularly in cats when treated with long-acting preparations. In such cases, the insulin should be changed to an intermediate-acting preparation. Injections of insulin into poorly perfused large subcutaneous fat deposits or scar tissue may be less well absorbed than injections into more lean areas where there is greater movement. Be wary of declining insulin requirements as these problems are addressed. Remember that some individual cats and dogs simply require high doses of insulin to achieve reasonable glycaemic control.
Step 4: Take action as indicated by step 3 (decrease insulin dose or switch to twice daily insulin injections). If insulin resistance has been identified consider potential concurrent disease in the light of the individual animal. Carry out routine screening laboratory analyses, proceeding with more investigative techniques depending on results. If a concurrent problem is not identified consider insulin antibodies or poor subcutaneous absorption. It is possible for animals to develop an immunological resistance to insulin preparations from species without identical insulin structure. Switching species of insulin will correct the problem. Administration of insulin subcutaneously does not necessarily imply absorption and poor subcutaneous absorption has been identified particularly in cats when treated with long-acting preparations. In such cases, the insulin should be changed to an intermediate-acting preparation. Injections of insulin into poorly perfused large subcutaneous fat deposits or scar tissue may be less well absorbed than injections into more lean areas where there is greater movement. Be wary of declining insulin requirements as these problems are addressed. Remember that some individual cats and dogs simply require high doses of insulin to achieve reasonable glycaemic control.
Caused by insulin therapy Caused by concurrent disorder
Impaired insulin Diabetogenic drugs/Stress absorption Insulin antibodies Hyperadrenocorticism Prolonged Somogyi Infection overswing (oral/urinary/gastrointestinal) Obesity/weight loss Hyper/hypothyroidism Metoestrus/Acromegaly Renal/cardiac/hepatic insufficiency Phaeochromocytoma Hyperlipidaemia Exocrine pancreatic insufficiency Glucagonoma Chronic pancreatitis
Table 1. Potential causes of insulin resistance
|
Caused by insulin therapy |
Caused by concurrent disorder |
|
Impaired insulin absorption |
Diabetogenic drugs/Stress |
|
Insulin antibodies |
Hyperadrenocorticism |
|
Prolonged Somogyi overswing |
Infection (oral/urinary/gastrointestinal)
Obesity/weight loss
Hyper/hypothyroidism
Metoestrus/Acromegaly
Renal/cardiac/hepatic insufficiency
Phaeochromocytoma
Hyperlipidaemia
Exocrine pancreatic insufficiency
Glucagonoma
Chronic pancreatitis |
| Figure 1. | 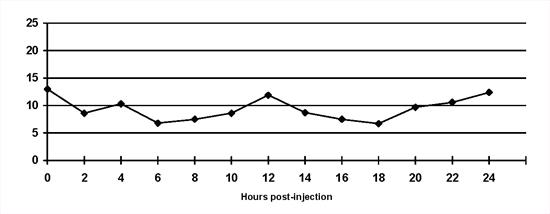
Normal blood glucose curve. Insulin is injected at 0 hours and 12 hours and the dog is fed half its meal at each time. The blood glucose concentration is maintained within the reference range (3.5 to 8.5 mmol/l) for most of the day. Blood glucose concentrations begin to rise as insulin activity wanes. |
|
| |
| Figure 2. | 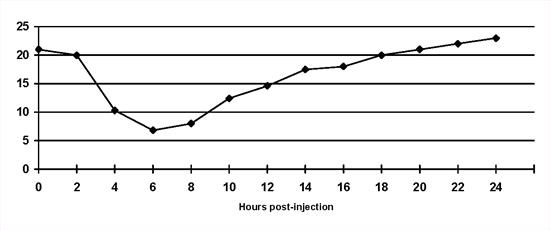
Short duration of insulin activity in a diabetic cat on once daily bovine lente insulin. Blood glucose concentrations escape from the effect of insulin 8 hours after injection. Note that a 'nadir' blood glucose concentration would have been within the reference range. |
|
| |
| Figure 3. | 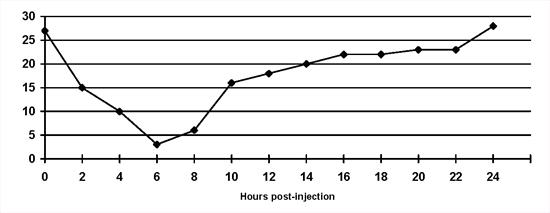
The Somogyi overswing in a dog on once daily porcine lente insulin. Note that the blood glucose concentration is inappropriately low at the 'nadir' point. |
|
| |
| Figure 4. | 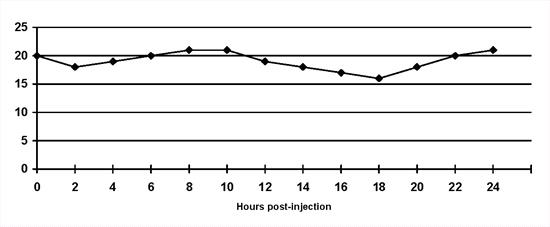
Insulin resistance: The blood glucose concentrations do not decrease despite twice daily lente insulin administration. |
|
| |
References
1. Briggs CE, Nelson RW, Feldman EC, Elliott DA & Neal LA (2000) Reliability of history and physical examination findings for assessing control of glycemia in dogs with diabetes mellitus: 53 cases (1995-1998). Journal of the American Veterinary Medical Association 217, 48-53.
2. Cassella M, Wess G & Reusch CE (2002) Measurement of capillary blood glucose concentrations by pet owners: a new tool in the management of diabetes mellitus. Journal of the AmericanAnimalHospital Association 38, 239-245.
3. Cohn LA, McCaw DL, Tate DJ & Johnson JC (2000) Assessment of five portable blood glucose meters, a point of care analyzer, and color test strips for measuring blood glucose concentration in dogs. Journal of the American Veterinary Medical Association 216, 198-202.
4. Crenshaw KL & Peterson ME (1996) Pretreatment clinical and laboratory evaluation of cats with diabetes mellitus: 104 cases (1992-1994). Journal of the American Veterinary Medical Association 209, 943-949.
5. Fleeman LM & Rand JS (2003) Evaluation of day-to-day variability of serial blood glucose concentration curves in diabetic dogs. Journal of the American Veterinary Medical Association 222, 317-321.
6. Hess RS, Saunders HM, Van Winkle TJ & Ward CR (2000) Concurrent disorders in dogs with diabetes mellitus: 221 cases (1993-1998). Journal of the American Veterinary Medical Association 217, 1166-1173.
7. Wess G & Reusch C (2000) Evaluation of five portable blood glucose meters for use in dogs. Journal of the American Veterinary Medical Association 216, 203-209.
8. Wess G & Reusch C (2000) Capillary blood sampling from the ear of dogs and cats and use of portable meters to measure glucose concentration. Journal of Small Animal Practice 41, 60-66.