Abstract
A number of wildlife species are known to accumulate excessive amounts of iron stores under conditions
of prolonged captivity.4 Recent studies of acquired hemochromatosis in browsing rhinoceroses5 were
expanded to determine whether this phenomenon also occurred in another browsing perissodactylid, Tapirus spp.
Fresh and frozen archival sera were obtained from seven Baird's (Tapirus bairdi), seven Malayan
(Tapirus indicus), and four mountain (Tapirus pinchaque) tapirs residing in U.S. zoos for an average of 13.0
yr (range: 0.7-27.0 yr). Sera from 13 adult Baird's tapirs free-ranging in Parque Nacional Corcovado, Costa Rica, obtained
over a 30-mo period up to February 2000, provided a comparison population of one species subsisting lifelong on natural
forage. Serum iron concentrations, total and unsaturated iron-binding capacities, and transferrin saturations were
determined by quantitative colorimetry. Serum ferritin concentrations were measured by the enzyme-linked immunoabsorbant
assay developed by Smith et al.6 using reagent standards derived from black rhinoceros ferritin which
cross-reacts with both equines and Tapirus spp.
In all three species of captive tapirs, mean serum iron concentrations were approximately twice normal
mammalian levels (165, 168, and 229.25 µg/dl for Malayan, Baird's, and mountain tapirs, respectively). Total iron
binding capacities were comparable in all three groups, but transferrin saturations were elevated to means of 60% (Malayan),
64% (Baird's), and 69% (mountain), again about twice normal mammalian levels. By contrast, mean values for 13 free-ranging
Baird's tapirs were 48.25 µg/dl iron with 33% transferrin saturation.
Serum ferritin assays, which provide the most reliable indirect measure of total-body iron stores, were
markedly elevated among all three captive tapir species, but individual values were scattered over a broad range of
concentrations. As shown in Figure 1, there was a general tendency for serum ferritin to increase progressively as a
function of time in captivity, and females appeared to have relatively greater concentrations than males. At comparable time
intervals, Baird's tapirs tended to show the highest ferritin values and Malayan tapirs the lowest.
Captive Baird's tapirs averaged 17,810 ng/ml (SD 17,130) for all seven animals, or 13,140 ng/ml (SD
2,580) if high and low outliers were excluded. In the captive Malayan and mountain tapirs, mean serum ferritin
concentrations were 8,740 ng/ml (SD 3,010) and 7,740 ng/ml (SD 4,100), respectively. By comparison, 13 free-ranging Baird's
tapirs had a mean serum ferritin content of 2,270 ng/ml (SD 1,110). Only one of seven captive Baird's tapirs had a ferritin
value that fell within the range exhibited by free-ranging Baird's tapirs. Mean ferritin concentrations for the free-ranging
tapirs and the entire captive population regardless of species differed significantly (P < 0.001).
This pattern of hyperferremia with increased transferrin saturation and marked elevation of serum
ferritin in captive tapirs closely resembled that which has been observed among captive populations of black5,6
and Sumatran rhinoceroses.5 Although iron analyte elevations in these tapirs were not as high as seen in many
captive rhinoceroses, their magnitudes were sufficient to raise concerns that chronic iron overloads, potentially leading to
clinically significant hemochromatosis, may be developing in tapirs maintained under conditions of prolonged captivity.
Since increased virulence of invading microorganisms is one of the principal consequences of free iron, its excess might be
contributing to the high incidence of infectious diseases affecting captive tapirs.2,3
Progressive iron loading in captive browsing rhinoceroses has been postulated to be caused by increased
bioavailability of dietary iron due to deficiencies of natural iron chelators that are normally present in native browse
forage, but are reduced or absent in formulated captive diets.5 This postulate may be applied to tapirs as well,
since they are also perissodactylids known to consume over 120 species of browse when foraging ad libitum in their natural
habitats.1
Quantitative trace metal analyses of necropsy tissues, reviews of histopathology with appropriate
stains, and development of species-specific reagents are needed to assess the clinical relevance of these serum analyte
measurements. Studies on iron homeostasis in this species may reveal the mechanisms responsible for iron overload, and
thereby provide strategies for prevention and/or treatment. Progressive accumulation of excess iron due to unregulated
dietary absorption may develop into clinically overt, acquired hemochromatosis, a problem apparently affecting an ever
widening range of wildlife species when brought into captivity.
| Figure 1. | 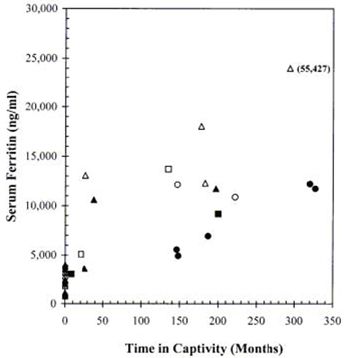
Serum ferritin concentrations in three species of captive tapirs as a function of
time in captivity compared to a population of free-ranging Baird's tapirs (zero time in captivity). |
|
| |
Baird's, mountain, and Malayan tapirs are represented respectively, by triangular, square, and circular
symbols (solid for males and open for females).
Acknowledgments
These studies were supported by grants from the International Rhino Foundation (D.E.P.) and the Zoological
Society of San Diego (S.H.F.)
References
1. Foerster CR, M McCoy. 2000. Comportamiento de forrajeo y dieta de una danta Centroamerican
en un bosque humedo tropical de Costa Rica. Vida Silvestre Neotopical (accepted, under revision).
2. Janssen DL, BA Rideout, ME Edwards. 1996. Medical management of captive tapirs
(Tapirus spp.). Proc. Amer. Assoc. Zoo Vet. Pp. 1-11.
3. Janssen DL, BA Rideout, ME Edwards. 1999. In: Fowler, M.E. and R.E. Miller (eds.). Zoo
& Wild Animal Medicine. Current Therapy 4. W.B. Saunders Co., Philadelphia, Pennsylvania. Pp. 562-568.
4. Lowenstine LJ, L Munson. 1999. Iron Overload in the Animal Kingdom. In: Fowler, M.E. and
R.E. Miller (eds.). Zoo and Wild Animal Medicine. Current Therapy 4. W.B. Saunders Co., Philadelphia, Pennsylvania.
Pp. 260-268.
5. Paglia DE, P Dennis. 1999. Role of chronic iron overload in multiple disorders of captive
black rhinoceroses (Diceros bicornis). Proc. Amer. Assoc. Zoo Vet. Pp.163-171.
6. Smith JE, PS Chavey, RE Miller. 1995. Iron metabolism in captive black (Diceros
bicornis) and white (Ceratotherium simum) rhinoceroses. J. Zoo Wildl. Med. 26: 525-531.