Non-obstructive Idiopathic/Interstitial Cystitis in Cats: Thinking Outside the (Litter) Box
Dennis J. Chew, DVM, DACVIM (Internal Medicine); C.A.T. Buffington, DVM, PhD, DACVN
Department of Veterinary Clinical Sciences, The Ohio State University College of Veterinary Medicine
Columbus, OH, USA
Introduction
A diagnosis of interstitial cystitis in people and cats requires identification of the presence of characteristic (although non-specific) sub-mucosal petechial hemorrhages-referred to as glomerulations) by cystoscopy, though the diagnostic value of this criterion is under debate. It is likely that the term idiopathic or interstitial cystitis in cats will be supplanted by more specific diagnoses as we improve our understanding of this frustrating syndrome. Results of studies over the past decade indicate that idiopathic cystitis in cats is the result of complex interactions between the bladder, nervous system, adrenal glands, husbandry practices, and the environment in which the cat lives (further detailed under pathophysiology).
Differential Diagnosis
Dysuria, stranguria, pollakiuria, macroscopic hematuria, and urinating in places other than the litterbox (inappropriate urination or periuria) are non-specific signs that, individually or in some combination, cause clients to bring their cats to a veterinarian due to apparent nonobstructive problems with the lower urinary tract regardless of the underlying cause. In cats less than 10 years of age, idiopathic cystitis accounts for clinical signs of irritative voiding in 60 to 70% of cats. Urolithiasis is encountered in 10 to 20% of cases with most, being associated with either calcium oxalate or struvite. About 10% may have an associated structural abnormality such as urachal diverticulum or urethral stricture, another 10% have what appears to be a behavior disorder, less than 2% of cases will be associated with urinary infection, and less than 1% can be expected to have bladder or urethral neoplasia. In cats older than 10 years of age at first presentation, only about 5% can be expected to be idiopathic. More than half of cats in this age category will have bacterial urinary tract infection, either alone or in association with urolithiasis. Many of these cats with positive quantitative bacterial cultures will have renal disease and sub-maximally concentrated urine.
Diagnosis
Idiopathic cystitis affects males and females equally, although neutered males and females are at increased risk compared to their intact counterparts. An affected cat typically is 1 to 10 years of age (peak risk 2-6 years), spends all or nearly all of its time living indoors with humans, is expected to use a litter pan for urination and defection, and eats 75 to 100% dry food. Obesity and a variety of other comorbid conditions may be associated with idiopathic cystitis. Owners sometimes note that affected cats are unusually nervous, fearful, or aggressive, and are overreactive to their environment compared to healthy cats. Cats with access to the outdoors still can be affected, especially when the cat population in the outdoor area is dense.
Abdominal palpation may reveal pelvic pain and/or thickening of the bladder wall in some affected cats. The bladder is usually small during active bouts of cystitis. The rest of the examination usually is normal.
Urinary tract imaging is recommended for all cats with recurrent LUTS. Survey radiographs are helpful to identify radiodense calculi such as calcium oxalate or struvite, which usually are observed if > 2-3mm in size. In those cats with multiple recurrences or persistence of clinical signs, advanced urinary imaging should be pursued to exclude radiolucent calculi and anatomical defects if the survey radiographs were normal. Abnormalities that can be identified during double-contrast cystography include focal or diffuse thickening of the bladder wall, permeation of contrast agent into the bladder wall or through the bladder and into the abdomen, and filling defects in the contrast pool (blood clots and cellular debris). Ultrasonography (ULS) can be a useful, less invasive method of imaging than contrast urethro-cystography. The proximal urethra can be examined with ULS, but ULS is not a good method to image the urethra, as most of the urethra cannot be examined. Cystoscopy (uroendoscopy), which provides direct visualization of the internal surface of the bladder, is available at some referral centers. Excellent evaluation of the urethra and bladder lumen usually is possible in female cats weighing at least 3 kg using a rigid pediatric cystoscope. The bladder of idiopathic cystitis cats will often display a varying degree of increased vessel density and tortuosity, edema, and sub-mucosal petechial hemorrhages (glomerulations). Increased number or size of glomerulations and increasing edema can be observed when higher bladder filling pressure (~80 cm water) is used during the scoping, findings that do not happen in cats with normal bladders.
Findings from urinalysis are useful, but are neither sensitive nor specific. The classical findings of hematuria and proteinuria in cats affected with idiopathic cystitis often wax and wane between days and even within the same day. Additionally, it is impossible to know with certainty that red cells and protein in the urine did not enter during collection when cystocentesis is performed. The classical positive finding is "hemorrhagic inflammation", which means that there is a preponderance of red blood cells with few neutrophils in the urine sediment. Crystals often are not present when fresh urine is evaluated. If crystals are observed, they usually are present in low numbers. Refrigeration can cause the formation of crystals ex vivo that were not present in vivo. Regardless, the presence of crystals has NO known diagnostic or pathophysiologic impact on non-obstructive forms of idiopathic cystitis. Struvite or calcium oxalate crystals do not damage a healthy urothelium. Conventional wisdom previously held that crystals formed and subsequently caused damage to the lower urinary tract, but it is more likely that sterile (neurogenic) inflammation occurs first, plasma proteins exude into urine, urinary pH increases, and then struvite crystals precipitate as a secondary event. It is physiologically normal to observe a few crystals in urinary sediment, especially when the urine is highly concentrated. The urine specific gravity (USG) in healthy cats should be greater than 1.025 in those eating mostly canned foods, and greater than 1.035 in those eating exclusively dry foods. In cats with LUTS and USG less than 1.025, some systemic disease (renal disease, renal failure, hyperthyroidism, diabetes mellitus) may be present that is interfering with the formation of more concentrated urine. Though not specifically studied, our impression is that cats with extremely high USG (1.060-1.080) are at higher risk for perpetuation of idiopathic cystitis once initiated if not transitioned to a therapy that produces a lower USG.
Pathophysiology
Idiopathic cystitis can be acute or chronic. Clinical signs associated with an initial or recurrent episode of idiopathic cystitis often resolve within about 7 days with or without treatment. Nearly 50% of cats with idiopathic cystitis will have recurrent signs within one year based on recent studies. It appears that most cats with recurrence have episodic signs of idiopathic cystitis, but some have persistent clinical signs that do not abate.
The pathophysiology of chronic idiopathic cystitis appears to involve complex interactions between multiple body systems. Abnormalities have been found in the bladder, nervous system, hypothalamic-pituitary-adrenal axis, and other body systems in cats with idiopathic cystitis. Histological changes, urothelial abnormalities, and decreased excretion of both total urinary GAG and a specific GAG, GP-51, have been identified in the bladders of cats with idiopathic cystitis. Histological changes generally are nonspecific, and may include an intact or damaged urothelium with submucosal edema, dilation of submucosal blood vessels with marginated neutrophils, submucosal hemorrhage, and sometimes increased mast cell infiltration. There is a paucity of neutrophilic infiltration, but there may be a minor increase in lymphoplasmacytic cells in the submucosa.
In the brain, a significant increase in tyrosine hydroxylase (TH) immunoreactivity (IR) has been in cats with idiopathic cystitis.Tyrosine hydroxylase is the rate-limiting enzyme of catecholamine synthesis. Chronic activation of the stress response system can increase TH activity in the LC, with accompanying increases in autonomic outflow. The increased THIR observed in the LC of cats with idiopathic cystitis may provide a clue to the observation that clinical signs follow a waxing and waning course in animals with this disease, and can be aggravated by environmental stressors. Increased plasma norepinephrine (NE) and CSF catecholamine concentrations and their metabolites have been documented in cats with idiopathic cystitis when measured during stressful situations. Increased noradrenergic outflow may alter urothelial permeability, increasing C-fiber activity, and activate local neurogenic inflammatory mechanisms. Increased epithelial permeability could permit constituents of urine to gain greater access to sensory afferent neurons in the bladder wall, which could result in increased sensory afferent firing and local inflammation.
| Figure 1. | 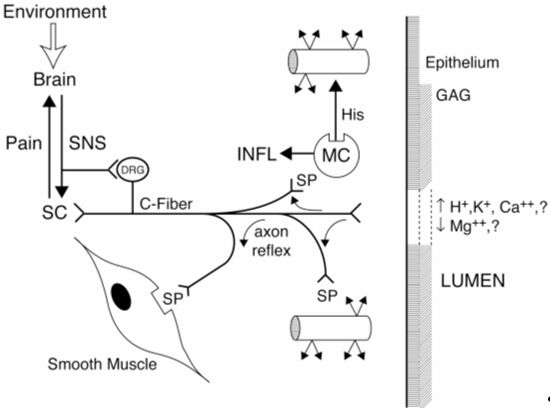
|
|
| |
Figure 1. Neurogenic inflammation as it affects the urinary bladder in interstitial cystitis.
Sensory neurons (C-Fiber) seem to play a central role in transmission of action potentials via the dorsal root ganglia (DRG) to the spinal cord (SC) and brain. These signals may be perceived as painful by the brain. Sensory fibers also can propagate a local axon reflex without transmission of an axon potential. The axon reflex results in release of peptide neurotransmitters such as substance P (SP) by the nerve endings. Interaction of SP with receptors on vessel walls results in vascular leakage, which can be augmented by SP-induced release of histamine by mast cells. These actions may give rise to the submucosal petechial hemorrhages (glomerulations) observed at cystoscopy. Receptors for SP also occur on smooth muscle, which when activated stimulate muscle contraction. Also shown are the urothelium (epithelium) and the overlying glycosaminoglycan (GAG) layer adjacent to the bladder lumen. Damage or malfunction of either or both of these layers may permit constituents of the urine, such as protons, potassium ions, or hyperosmolar (>2,000 mOsm/L) fluid to activate the sensory fibers. The effects of stress on sensory fibers may be related to descending efferent sympathetic (SNS) signals stimulating the DRG and inducing peripheral release of neuropeptides. Local release of neurotransmitters by bladder sympathetic fibers also could stimulate sensory fibers. Another factor probably involved in chronic, neurogenic inflammation of the bladder, but not shown, is local and systemic release of nerve growth factors, which may promote sensory fiber terminal sprouting to increase the size of sensory fiber receptive fields.
Abnormalities in the hypothalamic-pituitary-adrenal axis (HPA) also have been observed in cats with idiopathic cystitis. Increased concentrations of corticotropin releasing factor (CRF) from the hypothalamus and ACTH from the anterior pituitary gland have been identified at times of decreased serum cortisol response to ACTH stimulation during periods of stress in cats with idiopathic cystitis, documenting the presence of reduced adrenocortical reserve in this population. CRF stimulates both the release of ACTH from the anterior pituitary and activation of the sympathetic nervous system in the brainstem. During chronic stress in cats with idiopathic cystitis, there appears to be a disproportionate activation of noradrenergic outflow in the absence of a parallel increase in outpouring of adrenocortical steroids (ACS). This phenomenon may be important since cortisol and other ACS normally restrain sympathetic nervous system outflow, and also inhibit their own release by feedback inhibition at the level of the anterior pituitary and hypothalamus to terminate the stress response. Decreased ACS activity may adversely affect epithelial permeability, as cortisol is known to enhance tight junction integrity to reduce permeability in other tissues. However, cats with idiopathic cystitis do not appear to experience long-term benefit from current glucocorticoid therapy regimens.
Treatment
The waxing and waning natural history of cats with idiopathic cystitis has made it difficult to determine which treatments, if any, are effective. The goals of treatment are to decrease the severity and duration of signs during an acute episode (intra-episode), to increase the interval between episodes in those with recurrent idiopathic cystitis (inter-episode), and to decrease severity of signs in those with persistent idiopathic cystitis. Based on the pathophysiology described above, it is crucial to reduce the output of the sympathetic nervous system, since enhanced noradrenergic outflow appears to potentiate clinical signs by a variety of mechanisms. Based on the premise that cats with idiopathic cystitis are "sensitive cats in a provocative environment" one important objective of therapy is to identify and hopefully modify provocateurs (e.g., diet, water, indoor living with humans, sub-optimal husbandry, stress, and inactivity). Since chronic pain perception can amplify noradrenergic outflow, it is important to consider treatments that provide analgesia. Breaking the pain-inflammation cycle can be an important step in the management of some cats with chronic idiopathic cystitis. Providing analgesia systemically appears to be more important than analgesia within the bladder locally.
Treatment of a First Episode or an Infrequent Acute Flare
Resolution of clinical signs occurs in an estimated 85% of cats within one week, often without treatment, though the recurrence rate for clinical signs is high within the next 6 to 12 months with (or without) conventional treatment. Clinical signs for longer than 7 days are beyond the point of spontaneous resolution for most cats so specific recommendations are justified at that time.
Relief of bladder pain during acute episodes or flares of chronic idiopathic cystitis is recommended. Though not specifically studied, oral buprenorphine at 5 to 20 micrograms/kg BID to QID for 3 to 5 days has been helpful in providing relief to affected cats in our practice. Whether adequate provision of analgesia during acute episodes impacts development of future episodes currently is not known. The best regimen of analgesia for bladder pain (visceral) has yet to be determined.
Environmental Modification (EM)--Level 1
The overarching premise of EM is that some cats suffer adverse consequences of indoor housing, especially when cats are forced to spend nearly all of their time indoors in association with people and other animals. Ethological and behavioral studies demonstrate that captivity may elicit a stress response in some cats. The indoor environment of some house cats may be monotonous and predictable, which could be stressful. If we are to continue to recommend indoor housing to reduce the risks of exposure to accidents and infectious agents, recommendations to improve the indoor environment from the cat's point of view should be developed. Many indoor-housed cats appear to survive adequately by accommodating to less than perfect surroundings. The neuroendocrine abnormalities in cats with recurrent idiopathic cystitis suggest a sensitized response to stress indicating that these cats may have greater needs for enriched surroundings than do healthy cats. Extensive indoor housing in unenriched environments does not create idiopathic cystitis, but it can contribute to its development and maintenance by unmasking the tendency of a particular cat to develop idiopathic cystitis in response to external risk factors. Successful EM may obviate the need for drug therapy in many instances. Based on uncontrolled prospective studies at our hospital, we estimate that 80% of cats with recurrent idiopathic cystitis will have clinically significant reductions in signs during the year following successful implementation of the first level of EM. Stressors in an individual cat can emanate from another cat, people, other aspects of environment, or combinations of these.
Enhanced management of the litter box is essential for cats with idiopathic cystitis, and for those with "toileting issues". The goal is to make the litter box a pristine place for the cat to eliminate. Nothing should discourage the cat from frequent trips to the litter box--anything that discourages use of the litter box also may encourage longer periods of retaining urine between urinations. This may have adverse effects on cats with idiopathic cystitis, since longer periods of urine retention may facilitate constituents of urine gaining access to the bladder wall. Litter tray numbers, locations, cleaning schedule, substrate type, and the nature of the tray are all important areas for client education.
Some cats with idiopathic cystitis have extremely concentrated urine based on specific gravity (1.060-1.080), especially if they eat nearly exclusively dry formulations of commercial cat food. Transitioning to the highest percentage of canned food that the cat will eat, or adding water to dry food or to semi-moist food pouches, may be one of the most powerful single treatment recommendations for prevention of recurrence of signs of idiopathic cystitis. Adding water to pouches of semi-moist foods forms a gravy that many cats will consume before they eat the solid portion. Cats with recurrent idiopathic cystitis that consumed canned formulations of a veterinary diet compared to a similar formulation of the dry product had far fewer recurrences during one year of therapy. The benefit from the canned formulation might have resulted from a substantially lower USG compared to that of cats fed the dry formulation. The target USG is 1.030 or less to attempt to decrease recurrence of clinical signs. It is difficult to impossible to achieve this low a specific gravity in cats that continue to consume mostly dry food. Even when the desired target zone cannot be achieved, any reduction in USG has the potential to be helpful.
Dietary modification should be recommended mostly to increase water intake and decrease the concentration of noxious substances in urine as described above. However, some cats and owners prefer dry foods, and may become stressed by forced transition to canned foods. Attempts to further acidify urine and minimize struvite crystalluria often are not indicated, since no evidence supports the notion that struvite crystalluria damages normal urothelium or worsens existing cystitis in non-obstructive forms of idiopathic cystitis. Perhaps more important is maintaining the constancy, consistency, and composition of the diet that is being fed.
Intercat conflict commonly is present when multiple cats are housed indoors together and health problems are present. Conflict among cats can develop because of threats to the cat's perception of their overall status in the home, from other animals in the home, or from outside cats. The goal is to reduce conflict to a more manageable level for the cats involved. Treatment for conflict between indoor cats involves providing a separate set of resources for each cat, preferably in locations where the cats can use them without being seen by other cats.
Environmental modification also involves providing resources and interactions with and for cats to simulate some of the activities that they encounter in the wild. Simulations of prey, including laser light pointers, lures, and feathered fishing pole toys can provide useful interactions for some cats. Cats often enjoy playing with toys, particularly those that are small, move, and that mimic prey characteristics. Use of containers or toys that intermittently release food during play may provide actions to simulate hunting behavior.
Cats generally prefer more space than the average house or apartment provides. Cats interact with both the physical structures and other animals, including humans, in their environment. The physical environment should include opportunities for scratching (both horizontal and vertical may be necessary), climbing, hiding, and resting undisturbed. Cats seem to prefer to monitor their surroundings from elevated vantage points, so climbing frames, hammocks, platforms, raised walkways, shelves or window seats may appeal to them.
Synthetic feline facial pheromones are marketed to reduce urine marking or spraying behaviors in cats (Feliway; Ceva Sante Animale, Libourne, France). These pheromones reduce the vigilance of the cat so that the cat's need to mark or spray its territory is reduced. Since vigilance of cats is maintained largely by activity of the sympathetic nervous system, it is possible that use of these pheromones contributes to decreased adrenergic outflow from the brainstem in some cats. If so, they could be useful for treatment of chronic idiopathic cystitis in cats. A statistically significant effect could not be demonstrated in a study comparing facial pheromones and placebo in cats affected with idiopathic cystitis, though there appeared to be a positive trend. There appears to be a salutary effect of these pheromones in some cats (personal observations) and we continue to prescribe their use.
Further Environmental Modifications--Level 2
If implementation of EM at Level 1 does not adequately reduce signs of idiopathic cystitis, it is important to go back and review what was implemented and what was not, and why. Alternative approaches should be suggested for those that were not initially implemented based on collaboration with the client to address reasons for failure. Additional modifications also may be added at this time. Increased exposure to the outdoors can be helpful in the management of some cats.
Drug therapy is not attempted until analgesics have been administered and level-1 environmental modifications have been implemented without adequate resolution of clinical signs (including low-grade persistent signs, or frequent recurrence of clinical signs).
Tricyclic analgesics/antidepressants (TCA) can decrease clinical signs in some cats with recurrent idiopathic cystitis. Possible mechanisms include stabilization of mast cells (which may infiltrate the bladder wall during idiopathic cystitis), reduced contractions of detrusor muscle from anticholinergic effects, decreased sensory nerve pain fiber sensations from the bladder, effects on sodium, potassium, and glutamate channels, and downregulation of norepinephrine outflow from the brain. Two recent studies found no benefit of TCA for acute bouts of idiopathic cystitis; abrupt cessation of TCA administration after 7 days increased the severity of clinical signs and frequency of recurrence in one study. Prescription of amitriptyline was associated with resolution of clinical signs in 60 % of cats with severe recurrent idiopathic cystitis for one year during administration of 10 mg once daily by mouth at the owner's bedtime. Despite the decrease in clinical signs, no improvement in the cystoscopic appearance of the bladder mucosa was observed.. We prescribe TCA only when the EM treatments described above have not been sufficiently helpful. Cats may have a marked decrease in clinical signs of idiopathic cystitis during such treatment with amitriptyline, the TCA with which we have had the most experience. Despite the improvement in clinical signs, behavior of these cats may change, and weight gain and poor grooming may be noted by clients. We sometimes prescribe TCA while environmental modifications are being implemented. If EM is successful in reducing the cat's stress, it may be possible to taper the dose of TCA gradually and in some instances to stop this form of medication. Due to possible effects on liver enzymes or function during administration of TCA, we recommend a serum biochemical panel prior to starting the drug and again at 1,3 and 6 months during treatment. A complete blood count (CBC) is also recommended to ensure no adverse effects of chronic treatment are occurring (thrombocytopenia and neutropenia). TCA should be used cautiously if at all in cats with serious heart disease.
Studies to date have not shown a benefit of glucosamine or pentosan polysulfate (PPS) supplementation over that of placebo to cats with idiopathic cystitis. Whether GAG supplementation has a benefit in combination with other treatments, such as TCA or environmental modification, has not been investigated in cats. Diarrhea at routine doses and coagulopathy at high doses are possible side effects of GAG supplementation, but are rare.
References
1. Buffington CA, Westropp JL, Chew DJ, Bolus RR: Clinical evaluation of multimodal environmental modification (MEMO) in the management of cats with idiopathic cystitis. J Feline Med Surg 8(4): 261-268, 2006.
2. Buffington CA, Chew DJ, Woodworth BE: Feline interstitial cystitis. J Am Vet Med Assoc 215(5): 682-687, 1999.
3. Chew DJ, Buffington CA, Kendall MS, DiBartola SP, Woodworth BE: Amitriptyline treatment for severe recurrent idiopathic cystitis in cats. J Am Vet Med Assoc 213(9): 1282-1286, 1998.
4. Gunn-Moore DA, Shenoy CM: Oral glucosamine and the management of feline idiopathic cystitis. J Feline Med Surg 6(4): 219-225, 2004.
5. Gunn-Moore DA, Cameron ME: A pilot study using synthetic feline facial pheromone for the management of feline idiopathic cystitis. J Feline Med Surg 6(3): 133-138, 2004.
6. Kraijer M, Fink-Gremmels J, Nickel RF: The short-term clinical efficacy of amitriptyline in the management of idiopathic feline lower urinary tract disease: a controlled clinical study. J Feline Med Surg 5(3): 191-196, 2003.
7. Kruger JM, Conway TS, Kaneene JB, Perry RL, Hagenlocker E, Golombek A, Stuhler J: Randomized controlled trial of the efficacy of short-term amitriptyline administration for treatment of acute, nonobstructive, idiopathic lower urinary tract disease in cats. J Am Vet Med Assoc 222(6): 749-758, 2003.
8. Markwell PJ, Buffington CA, Chew DJ, Kendall MS, Harte JG, DiBartola SP: Clinical evaluation of commercially available urinary acidification diets in the management of idiopathic cystitis in cats. J Am Vet Med Assoc 214(3): 361-365, 1999.