M. Sc. Pablo Martínez Labat
SUMMARY
The infection produced by the nematode Toxocara canis is described, this one is a dog parasite; description also covers the factors associated to its presence, the way the parasite evolves inside the host, its pathogenic mechanisms, generated manifestations and prevention and treatment are as well considered.
Toxocara canis is a nematode parasite lodged inside the small intestine of young domestic dogs and wild ones; it is the cause of subclinical affection or serious and mortal cases. Larvae 2 (L2) live in visceral and muscle tissue of paratenics hosts, including man (many cases are no perceptible) causing the syndrome named larva migrans visceral and syndrome larva migrans ocular (Smith, 1993; Fisher, 2003; Rubel, et al, 2003).
Toxocara canis is widely found and it is very common no matters the geographic zone, its frequency ranges between 10 and 100%. Carriers of the adult form of this parasite release thousands of eggs in faeces, giving origin to infectious phase beings, which are keep viable in humid and shadow soil. Some places thousands of L2 helping an infectious state (Malloy and Embil, 1978; Martínez, 2004).
Adult dogs may keep susceptible to an intestinal infection and also work as paratenics hosts, female dogs carrying L2 for years in their bodies, are able to transmit larvae to their offspring though lactogénica and transplacental way, one that theses larvae are activated, they go from the mother host to its litter gradually, but only part of these larvae are activated and released in pregnancy and location, in the first pregnancy period the most severe infections are held in new born animals.
Another way for getting Toxocara infection in adult dogs is when they ingest L'2s at the moment they eat the bodies of paratenic hosts (rodents) or portion of their bodies (viscera and muscle tissue of big animals), due to this, the larvae concentration is constant and it varies according to sex, affecting animals health and their longevity (Kayes and Adams, 1976; Lee and Shu, 1976; Oliveira et al., 2002).
Human beings are terminal hosts, loading larvae in their visceras and skeletal muscles, infection occurs thought dirty hands or geophagia and it is more common in young children due to playing habits and high levels of contamination.
Human infection regularly affects young children, it is gradual and no evident in most of the cases having (it is also known as hidden toxocariosis), the cases having defined manifestations are known as visceral larva migrans syndrome, and also there is another varying where the parasite lodges in the eyes of the host, this is known as ocular larva migrans syndrome. This infection occurs all over the world having different prevalence levels, being Asiatic regions the main ones with 60-81.5%.
In México both ocular and visceral forms are reported, however these forms are considered underestimated and underdiagnosed (Gordon, 1984; Mancilla et al., 1992; Alba, 1999).
The development of this parasite varies according to the type of host, age and reproductive status. In dogs younger than 12 weeks, parasite development is similar to the other ascarids; ingesting eggs with L2, the larvae is released in the small intestine and migrates by tissue walls to vessels. Liver is reached and larvae pass through the gland having access to the blood once more, they reach heart and later the lung's parenchyma and be converted in the third larvae status. This last one gets bronchioli, bronchi, trachea and finally the pharynx where they are swallowing again. These larvae get the fourth and fifth larvae status in the stomach and intestine. Finally adult worm form are produced at 4 weeks, releasing eggs and staying here for several months before expulsion (Greve, 1971).
In paratenic host (dogs older 12 weeks or no dog's species and human beings) infestation occurs by larvae egg ingestion or encysted larvae in paratenic host. After ingestion larvae leave the egg or they are released from de tissues at the moment of digestion larvae leave the egg or they are released from of tissues at the moment of digestion. These larvae may cross the intestine wall, migrate by blood means and are derived by the stream to the whole organism becoming encysted in the brain an skeletal muscle, here, they may stay viable for many years; it has been found that the migration and dispersion patron is affected by the amount of larvae ingested by these hosts, besides, there is a strong evidence that this is an independent phenomenon of the immune response associated to previous infections in animals (Greve, 1971; Fox and Kasai, 1998; Helgwigh et al., 1999).
In female dogs, their pregnancy hormones reactivate the larvae during the second third, reaching the foetus via placenta and staying in the liver of these products up to being born. When puppies are born the larvae migrate to their lungs repeating the normal migratory behavior, having a shorter prepatent period due to a short distance to cover that also permits a fast access to the digestive app, to become mature (less than 15 days). In the same mother, larvae are reactivated and they migrate to the mammary gland, being released by calostrum, the larvae get inside offspring dogs developing a conventional migration. Transplacental migration has also been described in some other paratenic hosts such as mice (rural environment) (Lee et al., 1976).
In those cases where intestinal infection occurs the worms injure the host body while larvae migration destroying tissues, causing hemorrhages and hepatic and pulmonary inflammation with function deterioration. These disorders affect the liver in its metabolic capacity and respiratory performance. Later, the worms get the digestive tube having an important nutrient loss, altering the composition of the intestine mucosa causing hyperplasia of the covering layer of cells, some proteins are lost and hypertrophy of the smooth muscle layer. Sometimes one effect is vomit, obstructions and erratic migrations of the parasite having a lethal effect when this type of infection occurs (Fenoy et al., 2001).
Damaged caused by larvae depend on the number of organisms ingested, damaged tissue found, hemorrhages and chronic inflammation, granulomatous reactions in all visceral tissues (mainly brain), striated muscle and occasionally affected eyes, due to the lung survival of the larvae; inflammation is constant and causes larva isolation and this provokes a marked eosinophilia (values up to 50%) (Buendia, 2000; Fenoy, 2001; Oktar et al., 2002; Chia-Kung et al., 2003, Asao et al., 2003; Nobuaki et al., 2003).
Under lab conditions, experimental inoculation has show gradual development of leucocytosis with a serious eosinophilia, anorexia, depression, weakness, incoordination, motion damage and emaciation (Takur et al., 1998).
Infected humans beings show fever, eosinophilia up to 52%, leucocytosis as long as 15 months with persistence of high titres of antibodies kept up to 5 years long (Smith et al., 1953; Beaver et al., 1951; Tominura et al., 1976; Fenoy et al., 2003).
The damage associated to larvae infection aggravate when reinfection occurs, specially when the liver is affected where a temporary capsulate state goes by, the displacement due to inflammation is delayed, having this gland functions affected for a long time without meaning organ destruction (Parsons, !990; Fenoy et al., 2001).
Infected rodents suffer different states of alteration, facts that associated to the number of parasites present, and their distribution. These alterations show changes in behavior such as aggressivity, learning capacity, isolation, etc. It is also recognized that the mouse strain has influence in the clinical behavior during the infection; this fact considered under natural conditions may be translated as a probability raise level for depredation (Helwigh et al., 1999).
Slides show processed tissues by immune histochemical techniques containing Toxocara canis larvae.
| A. | 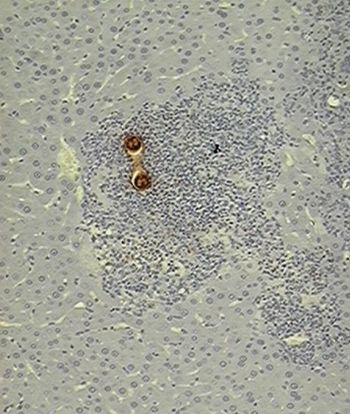
|
|
| |
|
A: The organism is surrounded by an inflammatory response from where the worm has capacity for evading since this gland is just a passing state (10X).
|
| B. | 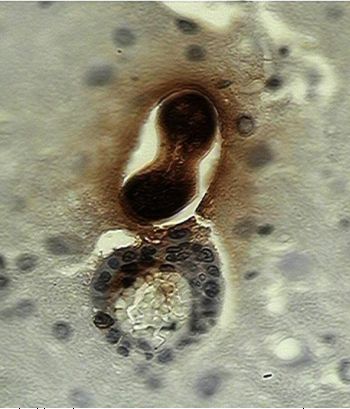
|
|
| |
|
B: There is a larva leaving a blood vessel in the brain, inflammatory cells accompany the worm. In both cases a dark brown staining is observed in larval and in cerebral tissue the diffusion of that material that corresponded to the secretion-excretion antigens (Photos Martínez, 2004).
|
Many disorders associated to larvae areas related to excretion-secretion products of the organisms which are antigenic and important in the host-parasite interaction, are important in the immune evasion processes (Glickman, 1979, Page et al., 1992).
In dogs, first manifestations of the infection are respiratory meaning dyspnea and nasal variable flow, that in many cases it may become severe with lethal results because bacteria or virus implication.
Manifestations of digestive app infection include: getting slimmer, altered hair, occasional vomit that may cause brocoaspiration, stretching abdomen and development delay. In human beings the typical visceral larva migrans syndrome range from to severe and the symptoms appears weeks or months later after infection; the presence of the parasite provokes alterations apparently related to allergic phenomena (coughing, anorexia, vomit, abdominal pain, hepatomegalia and also pneumonia, bronchitis and allergic respiratory manifestations, general discomfort and muscle pain, arthritis and pale mucose, besides there may be some nervous signs as headaches, confusion and progressive weakness (Vidal et al., 2003).
In the ocular presentation, there is a vision capacity lost (typically on one eye) endophthalmitis, peripheric granuloma, vitreus abbesses, optic neuritis, keratitis or uveitis (Bowman et al., 2000). Associated lesions are zonal hemorrhage and inflammatory reaction later, first as an acute type, later surrounded by fibrous material (specially in zones with low regeneration potential) with posterior encapsulation, the peripheric eosinophilic state is an important element and it also has diagnosis value (Tominura et al., 1976).
Helminthiasis is easily detected in puppies using coproparasitoscopic techniques or checking spontaneous elimination of mature or immature forms of the parasite.
In humans, most of the symptoms are not noticed or with slight manifestations (hidden form), those patients with visceral form manifestations may be detected by the ELISA and Western Blot tests. Ocular forms may be identified evaluating enzymatic systems or genetic factors for having a differentiation from retinoblastomas; besides ELISA technique also may be applied to the ocular fluids for specific anti-Toxocara antibodies (Yamasaki et al., 2000).
The prevention aspects are associated with puppies handling, which must be kept isolated from humans during the first weeks while they are not treated, loading cages must have water proof floors to be easily cleaned, cleaning must be done every day and also sodium hypochloride can be used. Treatments can be applied since the firsts four weeks of life, and it is recommended to have a second application at 2 and 4 weeks later to guarantee the complete elimination of the organisms (benzimidazoles, tetrahydropyridines, macrocyclic lactones) (Bowman et al., 2000).
For adult female dogs it is advisable to have periodic treatments at least every six months in order to prevent transplacentally and lactogenic transmission using drugs able to eliminate the worms (specially macrocyclic lactones) (Bowman et al., 2000).
In human beings the hygiene aspect must be considered, specially in children in their play yards, meaning hand washing, in order to eliminate contamination, stray animal contact must be avoided and the owned pets must be kept dewormed. General population must know the infectious status in order to be cautious so the health committee and small animal clinicians should give the proper information.
To fight toxocariosis there are a big variety of products with different formula mixtures and concentrations, having effect on adult forms and larvae , examples are dietilendiamidines, probencimidazolic drugs such as febantel, becimidazilic-carbamates such as mebendazole, albendazole, tetrahydropyridines such as pyrantel and oxantel tartrate or pamoate, imidazothiazoles such as levamisole, macrocyclic lactones such ivermectin, moxidectin and selamectin, besides diphenyl ethers such nitroscanate (Meyer, 1986; Fuentes, 1994; Sumano, 1997; Booth, 1998).
References
1. Alba H.F., (1999) Evaluación de un modelo de toxocariasis ocular y sistémica empleando jerbos (Meriones unguiculatus), Tesis doctoral, Facultad de Estudios Superiores Cuautitlán, UNAM.
2. Asao, N., Chu, A.E., Tsukidate, S., Fujita, K., (1997). A rapid and sensitive screening kit for the detection of anti-Toxocara larval ES antibodies. Parasitol.Int., 108, 184-195.
3. Beaver, P.C., Snyder, C.H., Carrera, G.M., Dent, J.H., Lafferty, J.W., (1951). Chronicaly eosinophilia due to visceral larva migrans. 65, 7-18.
4. Bowman D.D., Griffiths J.K., (2000). Larval Toxocariosis. Current Treatment Options in Infectious Diseases, 2: 70-77.
5. Buendía J. J.A., (2000). Evaluación de las lesiones histopatológicas producidas por larvas de Toxocara canis, en jerbos (Meriones unguiculatus) después del tratamiento con moxidectina, Tesis profesional, Facultad de Estudios Superiores Cuautitlán, UNAM.
6. Booth N. H. 1998. Farmacología y Terapéutica Veterinaria Vol. II. Ed. Acribia. España
7. Chia-Kwung, F., Yun-Ho, L., Wen-Yuan, D., Kua-Eire, S., Biers, B., Kimura, J.,(2003). Uveitis after death of a larva in the vitreus cavity. Am. J. Ophtal., 77)(1), 66-69.
8. Fenoy, S., Ollero, M.D., Guillén, J.L., Del Aguila, C.,(2001). Animal Models in ocular toxocariasis. J. Helmintol., 75, 119-124.
9. Fenoy, R.S., Cuéllar del Hoyo, C., Aguila, P., Guillén, (1992).Persistence of inmune response in human toxocariasis as measures by ELISA. J. Parasitol., 7, 1037-1038
10. Fisher, M., (2003). Toxocara cati: an underestimated zoonotic agent. Trends in Parasitol., 19(4) 167-169
11. Fox, E., Kassai, T., (1998).Toxocara canis infection in the paratenic host: a study on the chemosusceptibility of the somatic larvae in mice. Vet. Parasitol.,73, 244-259.-
12. Fuentes, M.A.. 1994. Farmacología Veterinaria. 2a ed. Ed. Interamericana McGraw-Hill. España.
13. Greve, J.H., (1971). Age resistance to Toxocara canis in Astrid free dogs. Am. J. Vet. Res., 32(8), 1185-1191.
14. Gordon, W., Green, J.A., Frothingham, T.E., Sturner, R.A., Walls, K.W., Patalnis, V.A., Ellis, G., S., (1984). Toxocara canis infection: Clinical and epidemiological associations with seropositivity in kindergrden children. J.Infect. Dis., 149(4), 591-596.
15. Glickman, L., Cypess, R., Hiles, D., Gessner, T., (1979). Toxocara-specific antibody in the serum and aquous humor of patients with presumed ocular an d visceral toxocariasis. Am.J.Trop. Med. Hyg. 28(1), 29-35.
16. Kayes, S.G., Adams, O.J., (1976). Effect of inoculum size and length of infection on the distribution of Toxocara canis larvae in the mouse. Am. J. trop. Med. Hyg., (25), 573-579.
17. Helwigh, A., B., Lind, P., Nanses, P., (1999). Visceral larva migrans: migratory pattern of Toxocara canis in pigs. Int. J. Parasitol., 29, 559-565.
18. Lee,K., Suh, Ch., (1976). Transplacental migration of Toxocara canis larvae in experimentally infected mice. J. Parasitol., 62(3), 460-465.-Meyer J.L. 1986. Farmacología y Terapéutica Veterinarias. Ed. Hispano-Americana. México.
19. Mancilla L.A.O., Llaguno V.N.P., Sánchez M.R.M.,(1994) Seroprevalencia de Toxocariosis en el hombre. Memorias del XI Congreso Nacional de Parasitología, Soc. Mex. Parasitol. 87.
20. Malloy, W.F., Embil, J.A., (1978). Prevalence of Toxocara spp and other parasites in dogs and cats in Halifax, Nova Scotia. Can. J. Comp., 42, 29-31.
21. Martínez L.J.P., Secuencia en el depósito de antígenos de decerción-excereción de larvas somáticas de Toxocara canis en tejidos de jerbos mongólicos (Meriones unguiculatus) con infección inducida, Tesis de Maestría, Facultad de Estudios Superiores Cuautitlán, UNAM, (2004).-Meyer J.L. 1986. Farmacología y Terapéutica Veterinarias. Ed. Hispano-Americana. México.
22. Nobuaki, A., Misado, T., Hayashi, E., Susuki, R., Masumi, Sh., Kasushiro, Sh., Koichiro, F., (2003). Cerebellar ataxia due to Toxocara infection in mongolian gerbils, Meriones unguiculatus. Vet. Parasitol., 113, 229-237.
23. Oktar, N., Barcin, E., Kazandi, A., Korkmaz, M., (2002). Cerebral Toxocara mimiking a malignant glioma. J. Neurol. Sc., 19, 1-4.
24. Oliveira-Sequeira, T.C.G., Amarante, A.F.T., Ferrari, T.B., Nunes, L.C., (2002). Prevalence of intestinal parasites in dogs from Sao Paulo State, Brasil. Vet. Parasitol., 75, 56-71.
25. Page, A.P., Hamilton, A.J., Maizels, R.M., (1992). Lectin binding to secretory structures, the cuticle and the surface coat of Toxocara canis infective larvae. Exp. Parasitol., 75, 72-86.
26. Parson, J.C., Grieve, R.V., (1990). Kinetics of liver trapping of infective in murine toxocariasis. J.Parasitol., 4, 529-536.
27. Parson, J.C., Coffman, .L., Grieve, R.B., (1993). Antibody to interleukin 5 prevent blood and tissue eosinophilia but not trapping in murine larval toxocariasis. Parasitol. Inm., 15, 501-508.
28. Rubel, , Zunino, G., Santillán, G., Winivesky, C., (2003). Epidemiology of Toxocara canis in the dog population from two areas of different socioeconomic status, Geater Buenos Aires, Argentina. Vet. Parasitol. 115, 275-286.
29. Smith, H.V., Kusel, J.L., Girdwood, W.A., (1983). The production of human A and Blood group like substances by in vitro maintained second stage Toxocara canis larvae: their presence on the outer larval surfaces and in the excretions-secretions. J. Clin. Exp.Inm.54, 625-633.
30. Sumano, H. 1997. Farmacología Veterinaria. Ed. Mc Graw-Hill; México.
31. Thakur, B.K., Murali, M.R., New, D., Smith, T.H., Volkman, D., (1998). Hipereosinophilia and markedly elevated immunoglobulin E in a 3 year old child. Ann. All., Asth., Inm.80, 371-376.
32. Tominura, T., Yolota, M., Takiguchi, H., (1976). I. Clinical, Hematological, biochemical and gross patological observations on monkeys inoculated with embrionated eggs of the domestic ascarid, Toxocara canis. Jap.J.Vet. Sc., 38, 533-548.
33. Vidal J.E., Sztajnbok J., Seguro A.C., (2003) Eosinophilic meningoencephalitis: Case roeport and review of the literatura, Am. J. Trop. Med. Hyg. 69 (3), 341-343.
34. Yamasaki H., Araki K., Kim Chooi Lim P., Zasmy N., Wah J.M., Aoki T., (2000). Development of a Highly Specific Recombinant Toxocara canis Second Stage Larva Excretory.Secretory Antigen for Inmunodiagnosis of Human Toxocariosis. J. Clin. Mic., 38(4), 1409-1413.