Introduction
The target for most traditional anti-cancer drugs is cellular DNA, and damage to DNA results in either failure of cell replication, and/or programmed cell death (apoptosis). Cytotoxic drugs are active against dividing cells. Some are cell cycle specific in their actions, that is to say they are only active against cells at one stage of the cell cycle, while some are active at all stages of the cell cycle. Cells that are resting (in G0) are not passing through the cell cycle (Figure 1) and are resistant to the effects of chemotherapy (and radiation) and these cells act as a reservoir of tumour cells, with which the tumour can repopulate after therapy.
| Figure 1. The cell cycle. | 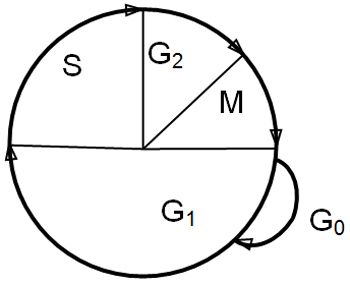
|
|
| |
The phases of the cell cycle are:
 M: mitosis. After mitosis, cells may either continue to cycle or pass into the resting phase, G0
M: mitosis. After mitosis, cells may either continue to cycle or pass into the resting phase, G0
 G1: intermitotic interval (enzymes and proteins required for DNA synthesis are produced)
G1: intermitotic interval (enzymes and proteins required for DNA synthesis are produced)
 S: synthesis phase (DNA synthesis)
S: synthesis phase (DNA synthesis)
 G2: premitotic phase (synthesis of RNA and protein, formation of the mitotic spindle)
G2: premitotic phase (synthesis of RNA and protein, formation of the mitotic spindle)
Tumour cells do not have intrinsically shorter cell cycle time than normal cells, but poorly differentiated tumour cells tend to complete the cell cycle more rapidly than well differentiated cells. The cell cycle time is independent of the stage of tumour growth. Tumours and normal tissues are composed of cells at all stages of the cell cycle and cells in G0.
Types of Anti-tumour Drugs
This is a brief description of the modes of action of drugs of various classes: readers contemplating using these agents should familiarise themselves with indications, dosages and toxicity profiles.
Alkylating Agents
These bind to DNA and substitute alkyl radicals for hydrogen atoms in the DNA molecule. This alkylation causes breaks, cross linkages and abnormal base pairing in the DNA, which interferes with DNA replication and transcription of RNA. These actions are not cell cycle stage specific. Commonly used alkylators are cyclophosphamide (metabolised to active form by liver), chlorambucil and melphalan, and lomustine (CCNU).
Plant Alkaloids
Include vincristine and vinblastine. Both prevent assembly of the mitotic spindle (by binding to microtubular tubulin) so their action is specific to the M phase of the cell cycle.
Anti-tumour Antibiotics
These include epirubicin, doxorubicin, mitoxantrone and actinomycin D. They inhibit DNA, RNA and protein synthesis by a variety of mechanisms dependent on binding to DNA, including intercalating into DNA, inhibiting topoisomerases II and creating free radicals that damage nucleic acids. Mitoxantrone produces much of its effect by inducing DNA strand breakages. The antibiotics tend to form very stable complexes with DNA and are very potent. Their actions are not cell cycle stage specific.
Antimetabolites
These either interfere directly with action of an enzyme, or result in the production of an aberrant molecule that cannot function correctly in cellular metabolism. Many are S phase specific, including the most commonly used agents cytosine arabinoside and methotrexate. Cytosine arabinoside is an analogue of 2'-deoxycitidine is incorporated into abnormal pyrimidine bases which can't stack normally in DNA. Cytosine also inhibits DNA repair enzymes. Methotrexate is a dihydrofolate reductase inhibitor: this enzyme is essential for synthesis of bases to make new DNA. Azathioprine, commonly used in immunosuppression in immune mediated disease, is in fact a purine analogue that inhibits enzymes involved in the early stages of purine synthesis, thus inhibiting DNA, RNA and protein synthesis.
Platinum Coordination Products
Inhibit protein synthesis by the formation or both inter- and intra-strand cross links in DNA. They also react with cellular proteins. They are cell cycle independent in their actions. Carboplatin is the most widely used agent. Cisplatin is rarely used because of the complexity of administration and safety issues.
Hormones
The only commonly used hormones in veterinary oncology are corticosteroids. Prednisolone and other corticosteroids bind to cytoplasmic receptors and inhibit DNA synthesis. They are lymphocytolytic and suppress mitosis in lymphoid cells. Sex hormone manipulation is rarely used in treatment of established tumours.
Enzymes
L-asparaginase hydrolyses asparagine, an essential amino acid resulting in death in cells that cannot synthesise asparagine. Most lymphoid tumour cells cannot synthesise asparagine, at least prior to exposure to L-asparaginase.
Tumour Growth: Some Important Definitions
Tumour growth fraction: the fraction of tumour cells that are actively dividing at any given time.
Mitotic index: the number of mitoses in the tumour assessed by high power light microscopy, expressed as number per examined field. (Includes only cells in the M phase, not in other phases of division.)
Mass doubling time: the time taken for the tumour to double in size. This depends on the growth fraction, cell loss factors and cell cycle time (see below).
In the early stages of tumour growth, the mass doubling time is short, but as the tumour grows this lengthens. This is largely due to a lower growth fraction, though there is also increased cell loss. The initial exponential phase of tumour growth occurs largely before the tumour is clinically detectable, and growth has slowed markedly by the time most cancers are detected. This pattern of growth is known as Gompertzian growth (Figure 2).
| Figure 2. Gompertzian growth. | 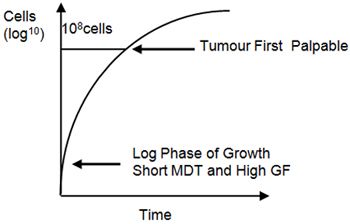
|
|
| |
Factors Affecting the Success of Chemotherapy
Growth fraction: most cytotoxic drugs act on dividing cells, so are most effective when the tumour is dividing rapidly. By the time most solid tumours are clinically detectable, the growth fraction is relatively low and there is therefore little chance of cytotoxic drugs being efficacious alone unless the tumour is inherently very chemosensitive or retains a high growth fraction (e.g., lymphoma).
Mass doubling time: tumours with a shorter mass doubling time are more chemosensitive than those with a longer mass doubling time: these smaller tumours have a high growth fraction.
Inherent tumour cell sensitivity: there is some variation in the response of tumours of different histogenesis to chemotherapy, and all individual tumour cells do not show the same sensitivity to drugs (see tumour cell heterogeneity).
Drug resistance: this may be either intrinsic or acquired in response to exposure to cytotoxic drugs. Exposure to one cytotoxic drug can induce resistance to that drug, or resistance to many different drugs: this is multidrug resistance.
Tumour cell heterogeneity: although it is convenient to consider that a tumour arises from one precursor cell, as the tumour grows the cells continuously modify due to mutations, giving rise to a heterogeneous population of cells, which may show considerable variation in their chemosensitivity. Additionally, metastatic deposits may not have the same chemosensitivity as the primary tumour.
Drug dosage: cytotoxic drugs kill cells by first order kinetics, that is any given dose kills a certain percentage of tumour cells. Even if a drug achieves 90% tumour cell kill, then it will reduce 108 cells to 107 cells, or 100 cells to 10 cells. In a lymphoma in complete remission (where cell kill may be as good as 99.9%) there are still many tumour cells left. Based on the cell kill hypothesis, more drug relative to less tumour is better. By using the highest possible dose, then the highest fractional kill will be achieved though this is limited by drug toxicity. By treating disease when tumour burden is minimal, tumour cell numbers remaining will be much reduced. In addition, the tumour growth fraction will be greater. However, even the most chemosensitive tumours will not be eradicated by a single treatment.
Tumour blood supply: this affects both the pattern of tumour growth and the efficacy of delivery of cytotoxic agents to the cancer cells. Large tumours tend to outgrow the blood supply so that there is inadequate drug delivery to tumour cells, which also have a low growth fraction. Where blood supply is poor, there may also be accumulation of metabolites that may alter the microenvironment and interfere with the action of the drug.
Principles of Clinical Use
Single Agent Therapy
This eliminates only the cells of the tumour that are sensitive to the agent, and will select for emergence of a repopulated tumour comprising cells which are resistant to that agent. It is likely that the first dose will achieve a better percentage kill than subsequent treatments as resistant cells are selected. In addition, these cells may show multidrug resistance.
Single agent chemotherapy is most often used where there are no other drugs of proven efficacy to add to the regime, or where combination with another effective drug would lead to unacceptable toxicity. In this latter situation, it is sometimes better to give the maximum tolerated dose of the most potent agent rather than reduce the dose sufficiently to allow concurrent administration of a less effective agent. Single agents are also used in rescue therapy, again as a result of a lack of other effective agents with which they can be combined.
Carboplatin (or cisplatin) is often used as a single agent in the treatment of osteosarcoma (micrometastatic disease), as is doxorubicin. This is because there are no other drugs of proven efficacy to add to these regimes, and concurrent use of both these agents is prohibitively toxic unless they were given on an alternating basis. However, no survival advantage has been shown using alternating agents.
Doxorubicin/epirubicin may be used as a single agent in the treatment of lymphoma and post-operative treatment of some sarcomas, which are likely to recur or metastasise as an alternative to the VAC protocol (vincristine, cyclophosphamide and doxorubicin).
Some haematological malignancies are routinely treated using single agents: polycythaemia rubra vera is treated with hydroxycarbamide (hydroxyurea), and chronic granulocytic leukaemia with busulphan.
Combination Chemotherapy
Should be more effective than single agent therapy, as this should combat the problem of inherent resistance in heterogeneous tumours, and make rapid selection of a resistant population less of a problem. In combining drugs, the following factors should be considered:
 Each agent must have proven efficacy against the tumour;
Each agent must have proven efficacy against the tumour;
 Agents should have different modes of action;
Agents should have different modes of action;
 Agents should affect different stages in the cell cycle;
Agents should affect different stages in the cell cycle;
 Agents should not have overlapping toxicities; and
Agents should not have overlapping toxicities; and
 Agents should not interfere with each others actions.
Agents should not interfere with each others actions.
Unfortunately, continued use of the same group of drugs (e.g., in maintenance therapy of lymphoma) will select resistant cells. Pulse therapy using different agents (ideally 3 groups of 3) and multidrug regimes as used in lymphoma are designed to further combat acquired resistance, but it remains a problem.
Dosage and Timing in Combination Chemotherapy
Cure is rarely achieved in veterinary chemotherapy as the dosages administered are limited to avoid unacceptable side effects, and are generally much lower than those in human oncology. Lowering drug dosages dramatically reduces tumour cell kill.
The interval between drug doses should be designed to allow recovery of the normal cell population, without being so long that there is recovery of the tumour cell population. This is important as a repopulated tumour is likely to contain more resistant clones. Treatment delays allow more tumour repopulation. Rapidly dividing tissue, such as bone marrow and the gastrointestinal tract (which are usually the limiting tissues in terms of toxicity), have a tremendous capacity to repair and generally achieve this more rapidly than tumours. If the interval between treatments becomes too short, then cumulative normal tissue toxicity will occur.
Minimising Resistance to Chemotherapy
Acquired resistance is exacerbated by misuse of drugs, especially improper dosing (which can be inadvertent due to drug mishandling), and improper time intervals. It can be minimised by treating as early as possible (as there is a less heterogeneous cell population), using standard protocols, correct dosing and correct drug preparation and administration, and correct scheduling. Animals with lymphoma must not be pretreated with corticosteroids prior to combination chemotherapy as this provokes early multidrug resistance.
Chemotherapy Combined with Surgery (or Radiotherapy)
 Post-operative adjunctive chemotherapy is the most common approach. The two aims are to treat micrometastatic disease after resection of the primary tumour and/or to treat microscopic residual tumour. After surgery, the residual tumour cells have a higher growth fraction and should be more sensitive to chemotherapy. This only applies to low volume disease: if you can see it, it's not going to be chemosensitive.
Post-operative adjunctive chemotherapy is the most common approach. The two aims are to treat micrometastatic disease after resection of the primary tumour and/or to treat microscopic residual tumour. After surgery, the residual tumour cells have a higher growth fraction and should be more sensitive to chemotherapy. This only applies to low volume disease: if you can see it, it's not going to be chemosensitive.
 Neo-adjunctive chemotherapy is occasionally utilised in veterinary medicine to 'down stage' tumours prior to surgery or radiotherapy. The advantages are easier surgery with a theoretically greater chance of achieving complete resection and earlier interception in metastatic disease.
Neo-adjunctive chemotherapy is occasionally utilised in veterinary medicine to 'down stage' tumours prior to surgery or radiotherapy. The advantages are easier surgery with a theoretically greater chance of achieving complete resection and earlier interception in metastatic disease.