Kit Sturgess, MA, VetMB, PhD, CertVR, DSAM, CertVC, MRCVS, RCVS Recognised Specialist in Small Animal Medicine
When to Intervene and What to Use
Chronic renal disease (CRD) leading to chronic renal failure (CRD) is a common finding in old cats (Figure 1). Whilst we are unable to reverse changes that have occurred, good management can lead to improved quality of life and slow the rate of disease progression.
| Figure 1. prevalence of chronic renal failure in cats. | 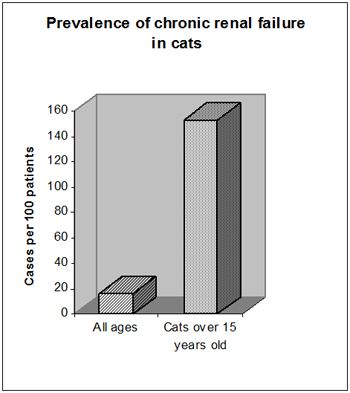
|
|
| |
In order to adequately manage a case of CRD it is necessary to obtain baseline haematological, biochemical and urinalysis data which can be used to decide on appropriate therapy and to evaluate the success of treatment. Nutritional management is a key element to successful therapy. Dietary intervention in cats with IRIS stage III & IV disease has been shown to increase median survival time from 266 to 633 days and is the only therapeutic intervention (apart from use of ACE inhibitors in proteinuric CRD) that has been shown to convey a survival advantage. Compared to many therapeutic interventions e.g., chemotherapy, the benefits are very significant and every effort should be made to encourage and support owners in appropriate nutritional therapy for cats with advancing renal disease. The value of nutritional therapy in cats with IRIS stage I & II disease is not proven although there are indications that it may be beneficial. It is helpful when discussing the management of renal disease to use a common system for describing the severity of the cases being managed.
IRIS Staging of Feline Renal Disease
The international renal interest society (IRIS) (http://www.iris-kidney.com/) has developed easy criteria for staging renal disease in cats and dogs. The primary method is based on creatinine estimation (Table 1) but further subdivision based on proteinuria and blood pressure have also been developed (Tables 2a and 2b).
Table 1. Staging based on creatinine.
|
Stage |
Plasma creatinine
(μmol/L [mg/dL]) |
|
I |
<140 [<1.6] |
|
II |
140-249 [1.6-2.8] |
|
III |
250-439 [2.9-5.0] |
|
IV |
>440 [>5.0] |
Table 2a. Substaging by proteinuria.
|
Urine protein: creatinine ratio |
|
Non-proteinuric (NP) |
<0.2 |
|
Borderline proteinuric (BP) |
0.2-0.4 |
|
Proteinuric (P) |
>0.4 |
Table 2b. Substaging by blood pressure.
|
Blood pressure (mmHg) |
|
Systolic |
Diastolic |
Comment |
|
<150 |
<95 |
Minimal risk (N) |
|
150-159 |
95-99 |
Low risk (L) |
|
160-179 |
100-119 |
Moderate risk (M) |
|
>180 |
>120 |
High risk (H) |
|
No evidence of end organ damage/complications (nc) |
|
Evidence of end organ damage/complications (c) |
Associated Problems
Problems associated with CRD that require management include
1. Dehydration and acidosis
2. Hyperphosphataemia
3. Raised urea and azotaemia
4. Hypokalaemia
5. Hypocalcaemia
6. Hypernatraemia
7. Vitamin B & C deficiency
8. Nausea and vomiting
9. Non-regenerative anaemia
10. Hypertension
11. Urinary tract infection
Dietary Management
Diet can have a major effect on problems 1-7 and a minor effect on problems 8-11 and can have a profound effect on the need for other medical intervention. In general, canned diets are preferred to dry diets as they have significantly higher water content, are generally more palatable and provide calories through fats rather than carbohydrates. The recommendations below represent the ideal in dietary modification. A significant number of cats will not tolerate such dietary modification. It is vital that the cat eats something, as body protein catabolism will have more serious adverse effect on CRD than almost any diet. If the ideal cannot be achieved, the recommendations below outline the basic aims of dietary modification.
When to Start Dietary Management?
Opinions differ as to the best time to start dietary management and the value of such management in early disease:
 Immediately azotaemia is identified
Immediately azotaemia is identified
 Set point of azotaemia, the consensus being BUN > 12-13mmol, creatinine > 250-300μmol/l
Set point of azotaemia, the consensus being BUN > 12-13mmol, creatinine > 250-300μmol/l
 When hyperphosphataemia develops
When hyperphosphataemia develops
Cats require 50-60 kcal/kg/day.
Dietary Phosphorus Restriction
Hyperphosphataemia has been found in around 60% of cats with CRD and has been correlated with the progression of renal failure, hence dietary phosphate restriction is crucial in the management of CRD (Figure 2). Additional phosphate restriction has been shown to blunt renal secondary hyperparathyroidism and reduce the level of PTH (implicated as an important uraemic toxin). Studies in both dogs and cats have suggested definite benefits from phosphate restriction, reducing mortality and improving well being. During the early stages of CRD low phosphate diets may be sufficient to control hyperphosphataemia. If, however, hyperphosphataemia persists in the face of dietary restriction, oral phosphate binders should be administered with food, aiming to maintain serum phosphate levels between 1.0-2.0 mmol/l (blood samples should be taken after a 12 hour starve to avoid post-prandial rises).
| Figure 2. Effect of diet on CDR in cats. | 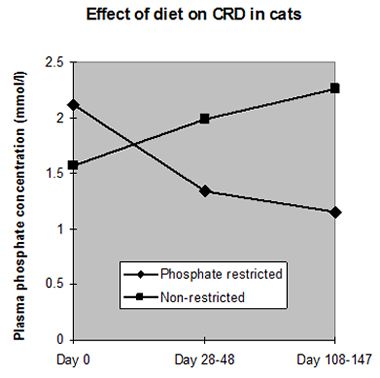
|
|
| |
Protein Restriction
Dietary protein restriction is associated with a fall in serum urea levels. This does not represent improved renal function but is a reflection of the change in nitrogen balance. Clinical observations suggest that in many cats CRD is slowly progressive and in some cases, renal function appears to remain stable for many months or years. In cats, when renal mass was reduced (sufficient to induce azotaemia), progressive decline in renal function was not observed, but feeding a protein restricted diet led to reduced glomerular hyperfiltration (reduced solute load), decreased proteinuria, and decreased histological glomerular damage compared to a group fed a high protein diet. From studies performed in dogs and cats, it can be concluded that there is no evidence in these species to suggest feeding high protein diets to normal animals is harmful. Even when azotaemia is established, evidence that protein restriction will benefit renal function is equivocal; nevertheless, studies have shown that protein restriction in the face of azotaemia results in amelioration of clinical signs of renal failure. It is generally recommended that protein should be restricted to approximately 20% of the total calories in CRD diets for cats but the optimum protein intake has not been established.
Management of Acidosis
Most renal diets are alkalinising, and supplementation without measuring acid-base status is potentially dangerous. Metabolic acidosis is a common complication affecting cats with CRD associated with a declining proton/bicarbonate exchange in the renal tubules. Initial compensation by an increase in ammoniagenesis (which may lead to further renal damage via complement pathways) and the excretion of protons as NH4+ is eventually overwhelmed. Metabolic acidosis may contribute to nausea, vomiting, lethargy, weakness, muscle wasting and weight loss seen in CRD. Chronically, acidosis may also promote the progression of renal failure; certainly feeding normal cats a poorly formulated, high protein, acidifying diet with inadequate potassium has been associated with inducing renal disease. Commercial diets for the management of CRD are designed to be non-acidifying. It is generally inappropriate to feed cats with IRIS stage III & IV disease acidifying diets designed to control urolith formation.
Fatty Acid Supplementation
It has been suggested that increased polyunsaturated fatty acids (PUFA) can lower the elevated cholesterol levels frequently seen in cats with CRD. Reduced cholesterol levels have been associated with preservation of renal function in dogs with experimental renal disease. However, it is possible that supplementation of diets with PUFA may increase renal damage associated with lipid peroxidation (degradation of carbon=carbon double bond by oxidants). The benefits over risks of increasing PUFA levels in the diets of cats with CRD have not been established. The ratio of omega-3 to omega-6 fatty acids may also be crucial as low ratios (0.02:1) have been associated with more rapid progression of renal disease in dogs when compared to high ratios (5:1). The beneficial action of omega-3 PUFA is thought to be due to their prostaglandin and thromboxane mediated effects on systemic hypertension and renal blood flow.
Energy Source
Dietary protein restriction is designed to limit the use of proteins as an energy source thereby reducing nitrogenous waste. Alternative energy sources are either carbohydrates or fats. Fats provide some advantages as they increase the caloric density of the ration which can be important in anorexic cats with CRD. Fats also increase dietary palatability which is often poorer in protein restricted diets. Amylase levels in cats are lower than those in dogs, hence high carbohydrate diets risk inducing diarrhoea (which can worsen dehydration) due to the presence of undigested carbohydrate in the lower small intestine and colon.
Management of Hypokalaemia
Cats with CRD often waste potassium in the urine in order to conserve sodium to maintain circulating volume. Wastage is further exacerbated by acidosis. In acidotic cats, there is an excess of hydrogen ions which have to be buffered. Some are taken into the cell to be neutralised there, but in order to maintain ionic balance across the cell membrane, the hydrogen ion is swapped with a potassium ion. This increases extracellular potassium leading to further wastage. CRD cats can therefore have a significant whole body potassium deficit. Hypokalaemia may increase renal ammoniagenesis which can cause further renal damage. Hypokalaemia causes an inflammatory myopathy which is seen initially as a stiff, stilted gait (often put down to arthritis in old cats) and a poor hair coat. More extreme hypokalaemia presents as muscle weakness and ventroflexion of the neck. Unfortunately, most potassium rich foods such as bananas and tomatoes are not very palatable to cats!
When to Start Supplementation?
Routine supplementation of all cats with CRD is not widespread. It is also important to remember that plasma potassium levels can be a poor reflection of whole body potassium status.
What to Use?
Potassium gluconate is most widely used as it is more readily accepted by cats. Some clinicians use potassium citrate, at least initially, in cats that are acidotic. Potassium chloride is not recommended as it is very acidic. Supplementation rates will vary according to the individual but generally 2-4mmol (mEq) per cat per day is required.
Management of Hypocalcaemia
In order to maintain a suitable calcium: phosphorus balance, most diets designed for the treatment of CRD in cats have lower calcium. Hypocalcaemia is usually associated with hyperphosphataemia, reduction in serum phosphate levels will naturally tend towards correcting the hypocalcaemia through reduced PTH levels. In those cats with persistent hypocalcaemia despite dietary management the use of vitamin D analogues should be considered.
Management of Hypernatraemia and Hypertension
Low salt diets possibly with an enhanced omega-3:omega-6 fatty acid ratio may help to reduce hypertension. However, neither the efficacy nor risks of salt restriction has been critically evaluated in cats with naturally occurring CRD as there is the potential for promoting pre-renal azotaemia, reducing palatability and promoting excessive renal potassium wasting. In experimental models, feeding high salt (2g/1000 kcal) did not show an adverse effect but feeding low salt (0.5g/1000 kcal) did activate neurohormonal mechanisms.
Vitamin Supplementation
Due to e increased turnover of water, cats with CRD tend to have a higher requirement for water soluble vitamins (B and C). Levels of supplementation tend to be increased in commercial diets designed for cats with CRD. If such diets are not used then oral supplementation is appropriate. Care should be taken if a vitamin/mineral preparation is used as many contain significant levels of calcium and phosphorus which may be undesirable. A specific vitamin supplement is preferred. Current recommendations for dietary inclusion rates of vitamin B and C are given below (Table 3).
Table 3. Suggested levels of intake of vitamin B and C in cats.
|
|
Per kg body weight
per day |
|
Vitamin B |
|
|
Choline |
120-130 mg |
|
Biotin |
1.5-3 μg |
|
Cobalamin |
0.3-1 μg |
|
Folate |
16-40 μg |
|
Niacin |
0.9-1.8 mg |
|
Pantothenic acids |
75-180 μg |
|
Pyridoxine |
70-200 μg |
|
Riboflavine |
90-320 μg |
|
Thiamine |
80-250 μg |
|
Vitamin C |
70mg |
Conclusions
Careful dietary formulation can address many of the problems associated with CRD and may be the sole method of therapy necessary in early disease. Considerable efforts towards finding a suitable diet that the cat will eat is justified as it can have a profound influence on the quality of a cat's life.
CRD is a dynamic and changing condition and good management will rely on periodic blood sampling but it is essential that any biochemical changes are interpreted in the light of the clinical picture. For example: a cat that is on a protein/phosphate restricted diet returns for blood sampling; urea and creatinine levels have fallen. If the cat has not been eating the diet, then urea levels will have fallen because of starvation, similarly weight loss has resulted in a reduced lean body mass and therefore creatinine (which is derived from muscle creatine) levels have fallen.
References
References are available upon request.